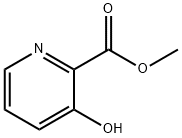
3-HYDROXY-PYRIDINE-2-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:3-HYDROXY-PYRIDINE-2-CARBOXYLIC ACID METHYL ESTER
- CAS Number:62733-99-7
- Molecular formula:C7H7NO3
- Molecular Weight:153.14

67-56-1
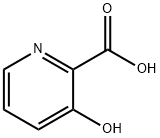
874-24-8

62733-99-7
A suspension of methanol (150 mL, 0.5 M) of 3-hydroxy-2-pyridinecarboxylic acid (10 g, 72 mmol, 1 eq.) was slowly added dropwise to concentrated sulfuric acid (12 mL, 216 mmol, 3 eq.) at 0 °C. The reaction mixture was stirred under reflux conditions for 6 hours. Upon completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure. Subsequently, the pH was adjusted to 8.5 with saturated aqueous NaHCO3 and solid NaHCO3. The aqueous layer was extracted with EtOAc, and the combined organic layers were washed with brine, dried over MgSO4, and concentrated under reduced pressure to afford methyl 3-hydroxy-2-pyridinecarboxylate as a white solid (10.9 g, 99%). Thin layer chromatography (TLC) showed Rf = 0.3 (petroleum ether/EtOAc 1:1, v/v). Melting point was 74°C. 1H NMR (300 MHz, CDCl3): δ (ppm) = 4.06 (s, 3H), 7.34 (dd, J = 3.9,8.4 Hz, 1H), 7.38 (dd, J = 1.5,8.4 Hz, 1H), 8.38 (dd, J = 1.5,3.9 Hz, 1H), 10.64 (s, 1H). 13C NMR (75 MHz, CDCl3): δ= 53.2,126.3,129.8,130.2,141.6,158.9,169.9. mass spectra (ESI+): m/z (%): 154 (100) [M + H]+.

67-56-1
790 suppliers
$7.29/5ml-f

874-24-8
302 suppliers
$14.14/1gm:

62733-99-7
170 suppliers
$9.00/250mg
Yield:62733-99-7 99%
Reaction Conditions:
with sulfuric acid at 0; for 6 h;Reflux;
Steps:
2.2.1. Synthesis of methyl 3-hydroxypicolinate (Compound 2)
To a suspension of 3-hydroxypicolinic acid 1 (10 g, 72 mmol, 1equiv) in methanol (150 mL, 0.5 M) was added dropwise at 0 °C aconcentrated sulfuric acid (12 mL, 216 mmol, 3 equiv.). The obtainedsolution was stirred at reflux 6 h. The cooled reaction mixture wasconcentrated under reduced pressure. The pH was adjusted at 8.5 withan aqueous solution of saturated NaHCO3 and solid NaHCO3. Theaqueous layer was extracted with EtOAc and the combined organiclayer was washed with brine, dried over MgSO4 and concentrated underreduced pressure to give 2 as a white solid (10.9 g, 99%). Rf=0.3(Petroleum ether/EtOAc 1/1, v/v). m.p. = 74 °C. 1H NMR (300 MHz,CDCl3): δ (ppm)=4.06 (s, 3H), 7.34 (dd, J=3.9, 8.4 Hz, 1H), 7.38(dd, J=1.5, 8.4 Hz, 1H), 8.38 (dd, J=1.5, 3.9 Hz, 1H), 10.64 (s, 1H).13C NMR (75 MHz, CDCl3): δ=53.2, 126.3, 129.8, 130.2, 141.6, 158.9,169.9. MS (ESI+): m/z (%): 154 (100) [M+H]+.
References:
Pashirova, Tatiana N.;Braïki, Anissa;Zueva, Irina V.;Petrov, Konstantin A.;Babaev, Vasily M.;Burilova, Evgenia A.;Samarkina, Darya A.;Rizvanov, Ildar Kh.;Souto, Eliana B.;Jean, Ludovic;Renard, Pierre-Yves;Masson, Patrick;Zakharova, Lucia Ya.;Sinyashin, Oleg G. [Journal of Controlled Release,2018,vol. 290,p. 102 - 111]

874-24-8
302 suppliers
$14.14/1gm:

62733-99-7
170 suppliers
$9.00/250mg
36851-80-6
6 suppliers
inquiry

874-24-8
302 suppliers
$14.14/1gm:

62733-99-7
170 suppliers
$9.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f
943777-92-2
5 suppliers
$378.19/1gm:

62733-99-7
170 suppliers
$9.00/250mg