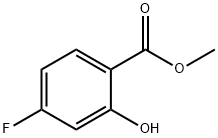
METHYL 4-FLUORO-2-HYDROXYBENZOATE synthesis
- Product Name:METHYL 4-FLUORO-2-HYDROXYBENZOATE
- CAS Number:392-04-1
- Molecular formula:C8H7FO3
- Molecular Weight:170.14

67-56-1
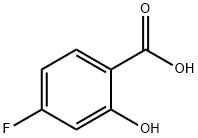
345-29-9

392-04-1
To a solution of 100 g (0.64 mol, 1.0 eq.) of 4-fluoro-2-hydroxybenzoic acid (XLIa) dissolved in 1 L of methanol at 0 °C was slowly added 100 mL of concentrated sulfuric acid. The reaction mixture was gradually warmed to room temperature and subsequently heated to reflux at 70 °C for 16 hours. Upon completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure to remove most of the methanol. The concentrated residue was redissolved in 1 L of ethyl acetate and washed sequentially with 500 mL of saturated aqueous sodium bicarbonate and 500 mL of saturated saline. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated in vacuum to afford 95 g (0.56 mol, 87% yield) of methyl 4-fluoro-2-hydroxybenzoate (XLIIa).
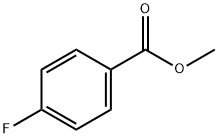
403-33-8
325 suppliers
$13.43/1gm:

392-04-1
139 suppliers
$24.64/1g
Yield: 78%
Reaction Conditions:
with oxone;Ru(MesCO2)(4,4'-dibromobipyridine)(p-cymene);trifluoroacetic acid;trifluoroacetic anhydride in 1,2-dichloro-ethane at 110; for 8 h;Sealed tube;Green chemistry;regioselective reaction;Reagent/catalyst;
Steps:
General procedure for ruthenium-catalyzed ortho-hydroxylation of aryl esters
General procedure: The Ru(MesCO2)(L) (p-cymene) [L- 2,2’-bypyridine or 4,4’-dibromobipyridine] (2.5 mol%), oxidant (2.0 eq) and ester (1.0 eq) were added to a sealed tube. Following that, trifluoroacetic acid (TFA) and trifluoroacetic anhydride (TFAA) in the ratio of 0.6 ml: 0.4 were added. The reaction mixture was kept on a pre-heated bath at 110°C and stirred until its completion. It was continuously monitored by TLC. Ice water was added to quench the reaction mixture and it was extracted with dichloromethane. The organic layer was dried over Na2SO4 and rota-evaporated. Finally the residue was purified by silica gel column chromatography to give corresponding products.
References:
Shome, Sanchari;Singh, Surya Prakash [Tetrahedron Letters,2017,vol. 58,# 38,p. 3743 - 3746] Location in patent:supporting information

345-29-9
232 suppliers
$10.00/1g

392-04-1
139 suppliers
$24.64/1g

345-29-9
232 suppliers
$10.00/1g

18107-18-1
211 suppliers
$33.00/5g

392-04-1
139 suppliers
$24.64/1g
