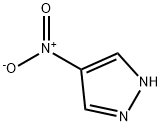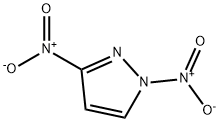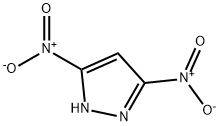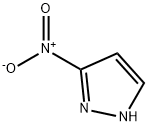
3-Nitro-1H-pyrazole synthesis
- Product Name:3-Nitro-1H-pyrazole
- CAS Number:26621-44-3
- Molecular formula:C3H3N3O2
- Molecular Weight:113.07
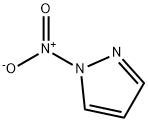
7119-95-1

26621-44-3
The general procedure for the synthesis of 3-nitro-1H-pyrazole from 1-nitropyrazole is as follows: Step A: 1-nitropyrazole (3.45 g, 30.5 mmol) was mixed with benzonitrile (33 mL) and heated with stirring at 180 °C for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature, diluted with hexane and stirring was continued for 20 min at room temperature. The precipitated solid was collected by filtration to afford 3-nitro-1H-pyrazole as a tan solid (3.16 g, 91% yield). Product characterization data: 1H NMR (300 MHz, DMSO-d6) δ 13.94 (br s, 1H), 8.03 (d, J = 2.4 Hz, 1H), 7.03 (t, J = 2.4 Hz, 1H).

7119-95-1
145 suppliers
$5.00/1g

26621-44-3
234 suppliers
$6.00/5g
Yield:26621-44-3 91%
Reaction Conditions:
in benzonitrile for 2 h;Reflux;
Steps:
A solution of 1 -nitro-1 H-pyrazole (4.23 gm, 37.4 mmol; Oakwood Products, Inc., West Columbia, SC) in benzonitrile (42 ml_) was heated to reflux for 2 hours. After cooling to 45 0C, the reaction mixture, which was starting to precipitate, was poured into 175 ml_ hexanes. A white solid precipitated. This was collected by vacuum filtration, rinsed repeatedly with hexane and dried under high vacuum to afford 3.85 grams (91 %) of (l-27a) as a white solid; 1H NMR (400 MHz, DMSO-d6) δ 13.90 (1 H), 8.01 (1 H), 7.01
References:
PFIZER INC.;BENBOW, John William;LOU, Jihong;PFEFFERKORN, Jeffrey Allen;TU, Meihua Mike WO2010/29461, 2010, A1 Location in patent:Page/Page column 63-64
1820-80-0
476 suppliers
$5.00/5g

26621-44-3
234 suppliers
$6.00/5g
288-13-1
456 suppliers
$10.00/1g

26621-44-3
234 suppliers
$6.00/5g