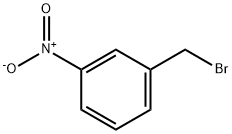
3-Nitrobenzyl bromide synthesis
- Product Name:3-Nitrobenzyl bromide
- CAS Number:3958-57-4
- Molecular formula:C7H6BrNO2
- Molecular Weight:216.03
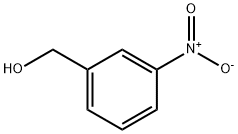
619-25-0

3958-57-4
General procedure for the synthesis of 3-nitrobenzyl bromide from 3-nitrobenzyl alcohol: Powdered 3-nitrobenzyl alcohol (0.5 mmol) was mixed with halosilanes (0.55 mmol) in a 4 mL screw-capped vial and stirred or heated at room temperature. The reaction mixture was kept at 70-75 °C for 0.5-24 h, during which the reaction progress was monitored by TLC. Upon completion of the reaction, the crude product was cooled to room temperature and the volatile by-product (TMS) was removed by evaporation under reduced pressure (30-35 °C).2O. The residue was confirmed by 1H NMR analysis. If necessary, the pure 3-nitrobenzyl bromide was purified by column chromatography on dry silica to obtain pure 3-nitrobenzyl bromide. Detailed experimental data, including isolated yields, spectral and other characterization data, are given in the Final Product Characterization section of the Supporting Information.

619-25-0
226 suppliers
$8.00/5g

3958-57-4
251 suppliers
$14.00/10g
Yield:3958-57-4 99%
Reaction Conditions:
with trimethylsilyl bromide in neat (no solvent) at 20; for 24 h;Green chemistry;chemoselective reaction;
Steps:
General procedure for the halo functionalization of organic compounds using halosilanes on half mmol scale
General procedure: A mixture of alcohol (0.5 mmol) in the case of solids, which had been powedered for 1-2 min and halosilanes (0.55 mmol) was transferred to a 4 mL screw-capped vial, and stirred at rt or heated at 70-75 °C for 0.5 h-24 h. The progress of the reaction mixture was monitored by TLC. Upon completion of the reaction, the crude reaction mixture was cooled down to the room temperature and volatile product (TMS)2O was removed by evaporation at 30-35oC under reduced pressure and the remaining was analysed by 1H NMR. Finally, if necessary, the pure final product was obtained after column chromatography on dried silica. Detailed experimental information such as isolated yields, and spectroscopic and other identification data are given in Characterization Data of Isolated Final Products chapter in the SI.
References:
Ajvazi, Njomza;Stavber, Stojan [Tetrahedron Letters,2016,vol. 57,# 22,p. 2430 - 2433] Location in patent:supporting information
99-08-1
231 suppliers
$5.00/5G

3958-57-4
251 suppliers
$14.00/10g
99-61-6
655 suppliers
$5.00/10g

3958-57-4
251 suppliers
$14.00/10g
![Benzene, 1-[(methoxymethoxy)methyl]-3-nitro-](/CAS/20210111/GIF/142509-31-7.gif)
142509-31-7
2 suppliers
inquiry

3958-57-4
251 suppliers
$14.00/10g
![Benzene, 1-[(ethoxymethoxy)methyl]-3-nitro-](/CAS/20210305/GIF/1058649-37-8.gif)
1058649-37-8
0 suppliers
inquiry

3958-57-4
251 suppliers
$14.00/10g