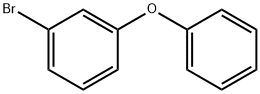
3-PHENOXYBROMOBENZENE synthesis
- Product Name:3-PHENOXYBROMOBENZENE
- CAS Number:6876-00-2
- Molecular formula:C12H9BrO
- Molecular Weight:249.1
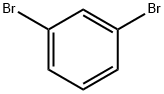
108-36-1

108-95-2

6876-00-2
Example 6 Synthesis of 1-bromo-3-phenoxybenzene (A): a mixture of meso-dibromobenzene (3 g, 12.75 mmol), phenol (1 g, 10.6 mmol), copper(I) oxide (152 mg, 1 mmol), and cesium carbonate (3.46 g, 10.6 mmol) was dissolved in 8 mL of N-methylpyrrolidone (NMP). The reaction mixture was microwaved at 195 °C for 20 min. Upon completion of the reaction, the non-homogeneous mixture was filtered through a bed of diatomaceous earth and the residue was washed with ethyl acetate (EtOAc, 1 × 20 mL). The filtrate was diluted with 1N sodium hydroxide solution (200 mL) and subsequently extracted with ethyl acetate (3 × 100 mL). The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give the crude product as a yellow oil. The crude product was purified by column chromatography (100% hexane as eluent) to afford 1-bromo-3-phenoxybenzene as a colorless oil (1.4 g, 53% yield).LCMS m/z 250 (M + 1).

108-36-1
455 suppliers
$10.00/1g

108-95-2
786 suppliers
$14.00/25g

6876-00-2
120 suppliers
$38.00/1g
Yield: 96%
Reaction Conditions:
with caesium carbonate;copper(II) oxide in 1-methyl-pyrrolidin-2-one at 195; for 0.833333 h;Inert atmosphere;
Steps:
107.1 Step 1: Synthesis of compound 1-bromo-3-phenoxybenzene
Dissolve m-dibromobenzene (3.9g, 17mmol) and phenol (1.3g, 14mmol) in N-methylpyrrolidone (45mL),Then copper oxide (0.11g, 1.4mmol) and cesium carbonate (5g, 15mmol) were added, protected by nitrogen, and heated to 195 ° C for 50min.Stop heating, cool to room temperature, filter through diatomaceous earth, and dilute with water (100 mL).Extract with ethyl acetate (80 mL × 3), collect the organic phase, dry over anhydrous sodium sulfate, filter, and concentrate.Silica gel column chromatography (PE) was separated and purified to obtain 3.4 g of light yellow liquid. Yield: 96%.
References:
Guangdong Dongyangguang Pharmaceutical Co., Ltd.;Liu Bing;Bai Shun;Yang Tiping;Zhou Youbo;Zhang Yingjun;Zheng Changchun;Luo Ming;Li Shixi;Peng Dahua;Wu Shuang;Xiong Jinfeng CN107344940, 2020, B Location in patent:Paragraph 1667-1672
89598-96-9
345 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6876-00-2
120 suppliers
$38.00/1g
591-20-8
349 suppliers
$10.00/1g
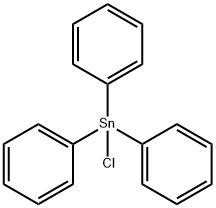
639-58-7
201 suppliers
$20.00/10 g

6876-00-2
120 suppliers
$38.00/1g
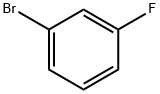
1073-06-9
448 suppliers
$6.00/10g

108-95-2
786 suppliers
$14.00/25g

6876-00-2
120 suppliers
$38.00/1g

591-20-8
349 suppliers
$10.00/1g
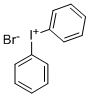
1483-73-4
129 suppliers
$32.00/5g

6876-00-2
120 suppliers
$38.00/1g