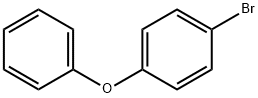
4-Bromophenoxybenzene synthesis
- Product Name:4-Bromophenoxybenzene
- CAS Number:101-55-3
- Molecular formula:C12H9BrO
- Molecular Weight:249.1
Yield:101-55-3 78%
Reaction Conditions:
with sodium dodecyl-sulfate;potassium carbonate in water at 80; under 760.051 Torr; for 8 h;Green chemistry;chemoselective reaction;Ullmann Condensation;
Steps:
General procedure for O-arylation of phenols with aryl halides
General procedure: To a stirred suspension of appropriate aryl halide(2.0 mmol) and phenol (2.0 mmol) in 6 ml water, Ni-alumina (0.125g, 6 mol % ofnickel metal) was added followed by K2CO3 (0.28 g, 2mmol) and SDS (0.04 g, 8 mol%). The reaction mixture was stirred for therequired period of time at 80°C till the reaction was complete (monitored withTLC). Then the reaction mixture was cooled to room temperature, ethyl acetate(20 mL) was added to dissolve the product and the catalyst was separated simplyby filtration. The residue (recovered catalyst) was thoroughly washed withEtOAc (4×5 mL) followed by water (2×10 mL). The aqueous reaction mixture was repeatedly extracted with ethyl acetate (3×5 mL). The combined organic extractswere washed with water (3× 10 mL) and dried over anhydrous Na2SO4.The crude product was obtained by removal of the solvent under reduced pressure which was furtherpurified by filtration chromatography on a short column of silica gel using1-4% ethyl acetate-hexane as eluent.
References:
Ghatak, Avishek;Khan, Sagar;Roy, Rimi;Bhar, Sanjay [Tetrahedron Letters,2014,vol. 55,# 51,p. 7082 - 7088] Location in patent:supporting information
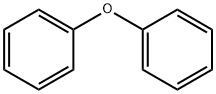
101-84-8
604 suppliers
$5.00/25g

101-55-3
251 suppliers
$8.00/5g
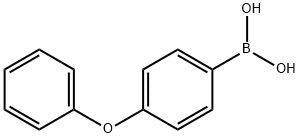
51067-38-0
352 suppliers
$9.00/1g

101-55-3
251 suppliers
$8.00/5g