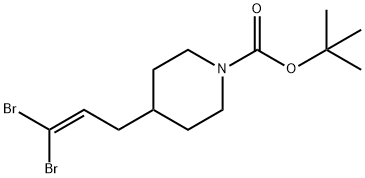
tert-butyl 4-(3,3-dibromoallyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(3,3-dibromoallyl)piperidine-1-carboxylate
- CAS Number:301185-40-0
- Molecular formula:C13H21Br2NO2
- Molecular Weight:383.12
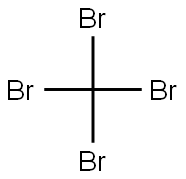
558-13-4
256 suppliers
$10.00/1g
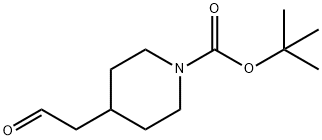
142374-19-4
122 suppliers
$12.00/100mg

301185-40-0
2 suppliers
inquiry
Yield:301185-40-0 118 mg (72%)
Reaction Conditions:
with triethanolamine;triphenylphosphine in dichloromethane;
Steps:
74.A Step A
Step A 1-(t-Butoxycarbonyl)-4-(3,3-dibromoprop-2-enyl)piperidine To a solution of 286 mg (0.86 mmol) of carbon tetrabromide in 4 mL of CH2Cl2 at -10° C. was added 339 mg (1.29 mmol) of triphenylphosphine. After 10 min, a solution of 98 mg (0.43 mmol) of ((1-t-butoxycarbonyl)piperidin-4-yl)acetaldehyde (from EXAMPLE 33, Step A) and 0.060 mL (0.43 mmol) of TEA in 2 mL of CH2Cl2 was added. After stirring at rt for 2 h, the reaction mixture was concentrated. The residue was purified by flash chromatography eluding with 9:1 v/v hexanes/EtOAc, then 1:1 v/v hexanes/EtOAc to give 118 mg (72%) of the title compound: 1H NMR (500 MHz) δ 1.14-1.22 (2H), 1.47 (s, 9H), 1.57-1.60 (m, 1H), 1.67 (br d, J=12.6,22H), 2.08 (t, J=7.1,22H), 2.70 (t, J=12.7,22H), 4.10 (br d, J=12.6, 2H), 6.42 (t, J=7.4, 1H).
References:
US6265434,2001,B1
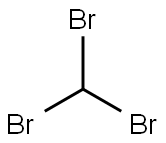
75-25-2
344 suppliers
$16.00/50g

142374-19-4
122 suppliers
$12.00/100mg

301185-40-0
2 suppliers
inquiry
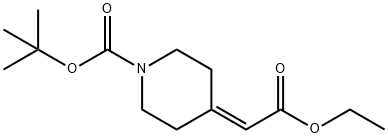
135716-08-4
141 suppliers
$5.00/250mg

301185-40-0
2 suppliers
inquiry

142374-19-4
122 suppliers
$12.00/100mg

301185-40-0
2 suppliers
inquiry
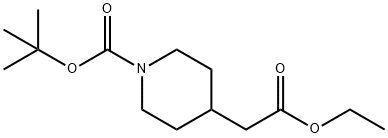
135716-09-5
110 suppliers
$9.00/1g

301185-40-0
2 suppliers
inquiry