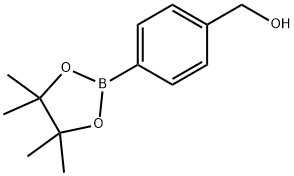
(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol synthesis
- Product Name:(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol
- CAS Number:302348-51-2
- Molecular formula:C13H19BO3
- Molecular Weight:234.1
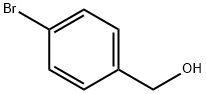
873-75-6

73183-34-3

302348-51-2
Using 4-bromobenzyl alcohol (compound 1, 1.0 g, 5.35 mmol) and pinacol ester of bisboronic acid (247 mg, 5.88 mmol) as raw materials, compound 1 was dissolved in 10 mL of anhydrous 1,4-dioxane under nitrogen protection, and potassium acetate and PdCl2 (Dppf) were added as catalysts. The reaction mixture was heated to 85 °C and stirred until the reaction was complete (monitored by thin layer chromatography). After completion of the reaction, the solvent was removed by evaporation under reduced pressure and the residue was extracted by adding ethyl acetate (200 mL) to the residue. The organic phase was dried with anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product. The crude product was purified by column chromatography (mobile phase: ethyl acetate/petroleum ether = 1:8 to 1:4) to afford 181 mg of a light yellow oily liquid, 4-(hydroxymethyl)benzeneboronic acid pinacol ester, in 90% yield.
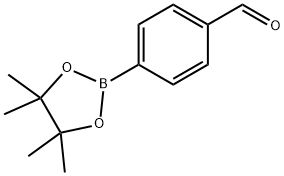
128376-64-7
189 suppliers
$5.00/250mg

302348-51-2
133 suppliers
$5.00/250mg
Yield:302348-51-2 513 mg
Reaction Conditions:
with sodium tetrahydroborate at 20; for 5 h;
Steps:
[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]methanol (2a).
To a mixture of a4-formylbenzenboronic acid (1a, 375 mg, 2.50 mmol), pinacol (355 mg, 3.00 mmol) and anhydrous magnesium sulfate (625 mg, 5.00 mmol), methanol was added (12.50 mL). The mixture was stirred at room temperature for 6 h. After the reaction was completed, the crude solution was filtered, and then sodium borohydride (47 mg, 1.25 mmol) was added to the filtrate. Afterwards, the reaction mixture was stirred for an additional 5 h. Once the reaction was completed, the reaction mixture was filtered and the filtrate was concentrated in vacuo to give the desired product 2a as a white solid (m.p. 75-77 °C) in88% yield (513 mg). 1H-NMR (CD3OD-d4) δ ppm 7.71 (d, J = 8.0 Hz, 2H), 7.35 (d, J = 7.8 Hz, 2H),4.62 (s, 2H), 1.34 (s, 12H); 13C-NMR (CD3OD-d4) δ ppm 146.23, 135.93, 127.26, 85.19, 65.24, 25.34;11B-NMR (CDCl3) δ ppm 34.82.
References:
Chung, Sheng-Hsuan;Lin, Ting-Ju;Hu, Qian-Yu;Tsai, Chia-Hua;Pan, Po-Shen [Molecules,2013,vol. 18,# 10,p. 12346 - 12367]

873-75-6
318 suppliers
$6.00/5g

73183-34-3
587 suppliers
$6.00/5g

302348-51-2
133 suppliers
$5.00/250mg
18282-51-4
214 suppliers
$10.00/1g

73183-34-3
587 suppliers
$6.00/5g

302348-51-2
133 suppliers
$5.00/250mg
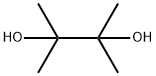
76-09-5
397 suppliers
$8.19/250mg
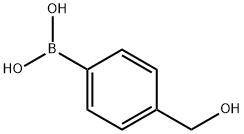
59016-93-2
319 suppliers
$5.00/1g

302348-51-2
133 suppliers
$5.00/250mg
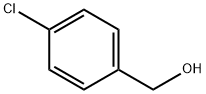
873-76-7
356 suppliers
$6.00/10g

73183-34-3
587 suppliers
$6.00/5g

302348-51-2
133 suppliers
$5.00/250mg