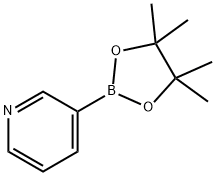
3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine synthesis
- Product Name:3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
- CAS Number:329214-79-1
- Molecular formula:C11H16BNO2
- Molecular Weight:205.06
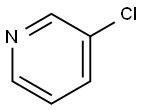
626-60-8

73183-34-3

329214-79-1
In a nitrogen-protected glove box, a benzene solution of silica-3p-TPP ([P] content 0.11 mmol/g, 45.5 mg, 0.005 mmol P, 1 mol% P), anhydrous degassed benzene (0.8 mL), and [PdCl(η3-cinnamyl)]2 (0.65 mg, 0.00125 mmol, 0.5 mol% Pd) (0.2 mL) was added sequentially to an oven-dried 10 mL glass tube pre-fitted with a magnetic stirrer. After stirring for 5 min, KOAc (147 mg, 1.5 mmol), bis(pinacolato)diboron (2,140 mg, 0.55 mmol) and 3-chloropyridine (63.3 mg, 0.50 mmol) were added sequentially. The reaction tube was sealed with a screw cap and then removed from the glove box, and the reaction was stirred at 25°C for 10 hours. After completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad (eluting with Et2O) and the filtrate was concentrated under reduced pressure. To the residue was added the internal standard 1,1,2,2-tetrachloroethane, and the yield of the product 3-pyridineboronic acid pinacol ester was 95% as determined by 1H NMR. The crude product was purified by silica gel column chromatography to afford the target compound 3-pyridineboronic acid pinacol ester (87.0 mg, 0.40 mmol, 80% yield).
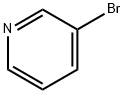
626-55-1
573 suppliers
$5.00/5 / G

73183-34-3
586 suppliers
$6.00/5g

329214-79-1
242 suppliers
$6.00/1g
Yield:329214-79-1 95%
Reaction Conditions:
with PdCl2(dppf);potassium acetate in toluene at 100; for 3 h;
Steps:
I.2.3; I.5.3; I.6.4; III.12.3 Core side 1-I-2 Synthesis
3-Bromopyridine (130 g, 822.8 mmol)(250.73 g, 987.3 mmol) and 4,4,4 ', 4', 5,5,5 ', 5'-octamethyl-2,2'-bi (1,3,2-dioxaborolane) and PdCl2 (20.16 g, 24.7 mmol)And potassium acetate (242.24 g, 2,468.4 mmol) was added to a round bottom flask,Toluene (4,000 mL)And the mixture was stirred at 100 ° C for 3 hours.When the reaction is terminated, the reaction product is quenched by adding water, and water in the reaction product is removed. After filtration under reduced pressure,The organic layer was dried over MgSO4 and concentrated to give 160.28 g (yield: 95%) of the product.
References:
Duksan Neolux Co.,Ltd.;Yoon Jin-ho;Jeong Ho-yeong;Park Mu-jin;Kim Jeong-seok;Lee Seon-hui KR2018/128292, 2018, A Location in patent:Page/Page column 22; 23; 25; 26; 40

626-60-8
369 suppliers
$13.00/5g

73183-34-3
586 suppliers
$6.00/5g

329214-79-1
242 suppliers
$6.00/1g
76-09-5
397 suppliers
$8.19/250mg
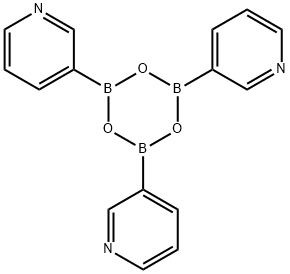
160688-99-3
5 suppliers
inquiry

329214-79-1
242 suppliers
$6.00/1g

626-55-1
573 suppliers
$5.00/5 / G

76-09-5
397 suppliers
$8.19/250mg
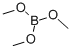
121-43-7
381 suppliers
$13.00/25mL

329214-79-1
242 suppliers
$6.00/1g
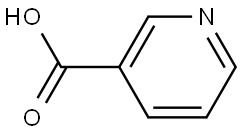
59-67-6
1145 suppliers
$7.00/25g

73183-34-3
586 suppliers
$6.00/5g

329214-79-1
242 suppliers
$6.00/1g