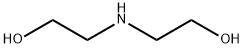
4-(2-hydroxyethyl)morpholin-3-one synthesis
- Product Name:4-(2-hydroxyethyl)morpholin-3-one
- CAS Number:41036-01-5
- Molecular formula:C6H11NO3
- Molecular Weight:145.16
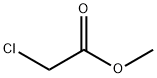
96-34-4
365 suppliers
$10.00/25g

41036-01-5
12 suppliers
inquiry
Yield:41036-01-5 80%
Reaction Conditions:
Stage #1: 2,2'-iminobis[ethanol]with potassium tert-butylate in toluene at 75; for 1 h;
Stage #2: methyl chloroacetate in toluene at 75; for 2 h;
Steps:
1; 3
Example 1 : Production of 4-(2-hvdrOxyethvDmorpholin-3-one (IV)Potassium tert-butoxyde (176 g, 1.1 eq.) was added to 1440 ml of toluene. The suspension was heated to 75 0C and maintained for 30 minutes until complete dissolution of the white solid. At this temperature diethanolamine (150 g, 1 eq.) was slowly added. The thick pale yellow suspension was maintained with strong stirring for 30 minutes and methyl chloroacetate (163 g, 1.05 eq.) was added slowly. The solution was maintained at the same temperature for two hours. To the warm mixture methanol (600 ml) was added and cooled at room temperature, salts were filtrated and the organic layer concentrated until dry. Compound (IV) was obtained as an orange oil (204 g, 98%) which was distilled under high vacuum to obtain it as a highly pure colorless oil (80%, bp5 18O0C). IR (film) (n cm-1 ): 3410, 2934, 2874, 1633, 1501 , 1350, 1141. MS (El), (m/z, %): 145 (M+., 12), 114 (M-CH2OH, 100), 86 (M-NC2H4OH, 65), 74 (M-71 , 7), 56 (M-89, 41), 42 (M-103, 44).; Example 3: Production of oxazolidinr2,3-c1morpholine (II) in a one-pot reaction starting from diethanolaminePotassium tert-butoxyde (176 g, 1.1 eq.) was added to 1440 ml of toluene. The suspension was heated to 750C and maintained for 30 min. until complete dissolution of the white solid. At this temperature diethanolamine (150 g, 1 eq.) was added slowly. The thick pale yellow suspension was maintained with strong stirring for 30 min. and methyl chloroacetate (163 g, 1.05 eq.) was added slowly. The solution was maintained at the same temperature for two hours. Reaction mixture was cooled at 30 0C and Vitride solution (412 g, 2 eq., 70% in toluene) was added slowly at room temperature. The reaction was maintained for 30 min at this temperature. 50% aqueous sodium hydroxide solution (360 g, 3.15 eq.) was added slowly keeping the mixture at room temperature. The mixture was warmed to 500C and the aqueous layer was separated and extracted at the same temperature with toluene (924 ml). Both organic layers were concentrated together until dry. Oxazolidine (II) was obtained (147 g, 80%) as a brownish oil.
References:
WO2007/57681,2007,A1 Location in patent:Page/Page column 8; 9
![3-Morpholinone, 4-[2-(phenylmethoxy)ethyl]-](/CAS/20210305/GIF/892871-54-4.gif)
892871-54-4
0 suppliers
inquiry

41036-01-5
12 suppliers
inquiry
622-40-2
363 suppliers
$5.00/25g

41036-01-5
12 suppliers
inquiry

186294-83-7
0 suppliers
inquiry

41036-01-5
12 suppliers
inquiry