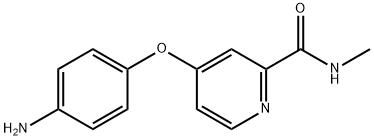
4-(4-Aminophenoxy)-N-methylpicolinamide synthesis
- Product Name:4-(4-Aminophenoxy)-N-methylpicolinamide
- CAS Number:284462-37-9
- Molecular formula:C13H13N3O2
- Molecular Weight:243.26
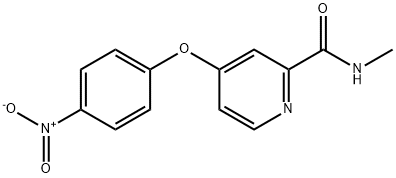
864272-34-4
15 suppliers
inquiry

284462-37-9
380 suppliers
$8.00/1g
Yield:284462-37-9 97%
Reaction Conditions:
with hydrogen;nickel in tetrahydrofuran;methanol at 20;Product distribution / selectivity;
Steps:
a.c
c) 3.7 g (17.11 mmol) 136 are hydrogenated with 2 g Raney-Ni in THF/MeOH at room temperature. After customary workup, 3.1 g (97 %) 25 (Rt. = 0.64 min (method C) ) is obtained as orange solid.
References:
WO2005/82853,2005,A1 Location in patent:Page/Page column 209
73771-11-6
87 suppliers
$45.00/100mg

284462-37-9
380 suppliers
$8.00/1g
99586-65-9
244 suppliers
$14.00/5g
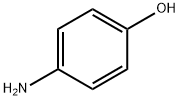
123-30-8
621 suppliers
$10.00/5g

284462-37-9
380 suppliers
$8.00/1g
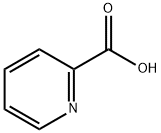
98-98-6
631 suppliers
$5.00/10g

284462-37-9
380 suppliers
$8.00/1g
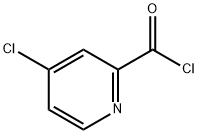
53750-66-6
102 suppliers
$60.00/250mg

284462-37-9
380 suppliers
$8.00/1g