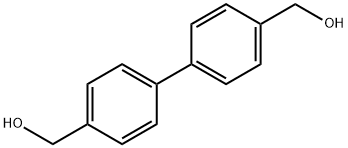
4,4'-Bis(hydroxymethyl)biphenyl synthesis
- Product Name:4,4'-Bis(hydroxymethyl)biphenyl
- CAS Number:1667-12-5
- Molecular formula:C14H14O2
- Molecular Weight:214.26
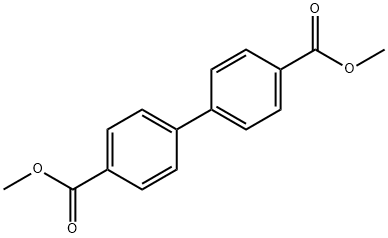
792-74-5

1667-12-5
a) Preparation of 4,4'-bis(hydroxy-methyl)biphenyl. In a 2-liter flask equipped with a mechanical stirrer, condenser, addition funnel and heating bath, 789 mg (20.8 mmol) of LiAlH4 was dissolved in 150 ml of anhydrous THF (tetrahydrofuran). A solution of 7.5 g (27.7 mmol) of 4,4'-bis(methoxycarbonyl)biphenyl dissolved in 500 ml of anhydrous THF was heated to 50 °C under an inert atmosphere and added dropwise to the above mixture over 1 hour. After the dropwise addition, stirring was continued at 50 °C for 30 minutes. Subsequently, the reaction mixture was cooled to room temperature and 15 ml of THF/H2O (80:20) mixture was carefully added to quench the excess LiAlH4. The reaction mixture was filtered through a Septum G2 filter and the precipitate was washed with ethanol (3 x 100 ml). The filtrate and washings were combined and evaporated to dryness under vacuum in a heating bath at 40 °C. The residue was redissolved in 100 ml of hot acetone (50 °C), filtered to remove insoluble matter, dried with anhydrous Na2SO4 and evaporated again to dryness. Finally 5.74 g of 4,4'-bis(hydroxymethyl)biphenyl was obtained in 96.6% molar yield.

792-74-5
243 suppliers
$14.14/1gm:

7732-18-5
507 suppliers
$12.69/100ml

1667-12-5
120 suppliers
$7.00/250mg
Yield: 96.6%
Reaction Conditions:
with LiAlH4 in tetrahydrofuran;ethanol;acetone
Steps:
2.a a)
a) Preparation of 4,4'-bis (Hydroxy-Methyl) Biphenyl 789 mg (20.8 mmoles) of LiAlH4 in 150 ml of anhydrous THF (tetrahydrofuran) are placed in a 2-liter flask which has a mechanical agitator, cooler, filler funnel and heating bath. A solution, heated to 50° C., of 7.5 g (27.7 mmoles) of 4,4'-bis (methoxycarbonyl)biphenyl in 500 ml of anhydrous THF is added dropwise, over a period of 1 hour, to the mixture, heated in an inert atmosphere to 50° C. under agitation. After a further 30 minutes of agitation in a heating bath, the mixture is cooled to ambient temperature and the excess LiAlH4 is destroyed by the careful addition of 15 ml of a THF/H2 O (80:20) mixture. The reaction mixture is filtered on Septum G2, washing the precipitate with anydrous ethanol (3*100 ml). The combined filtrate and washings are evaporated to dryness under vacuum with the heating bath at 40° C. The residue is then redissolved with 100 ml of hot acetone (50° C.), eliminating the insoluble residue by filtration, dried on anhydrous Na2 SO4 and evaporated to dryness. 5.74 g of 4,4'-bis (hydroxymethyl) biphenyl are obtained with a molar yield of 96.6%. Analysis: Melting Point: 195.8°-196° C. 1 H-NMR (DMSOD6): δ4.53 (d, 4H, CH2); 5.20 (t, 2H, OH); 7.35-7.65 (8H, aromatic) MS-EI: (M+)214; m/e 196, 183, 167, 165, 155, 152, 77.
References:
Eniricerche S.p.A. US5166380, 1992, A

792-74-5
243 suppliers
$14.14/1gm:

1667-12-5
120 suppliers
$7.00/250mg
59016-93-2
320 suppliers
$5.00/1g

1667-12-5
120 suppliers
$7.00/250mg
787-70-2
392 suppliers
$8.00/5g

1667-12-5
120 suppliers
$7.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1667-12-5
120 suppliers
$7.00/250mg