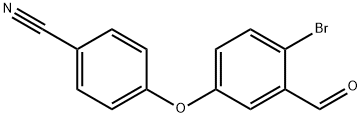
4-(4-Bromo-3-formyl-phenoxy)-benzonitrile synthesis
- Product Name:4-(4-Bromo-3-formyl-phenoxy)-benzonitrile
- CAS Number:906673-54-9
- Molecular formula:C14H8BrNO2
- Molecular Weight:302.12
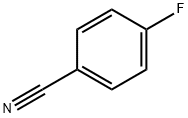
1194-02-1
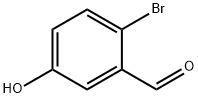
2973-80-0

906673-54-9
Under nitrogen protection, 2-bromo-5-hydroxybenzaldehyde (100 g, 497 mmol) and 4-fluorobenzonitrile (301.2 g, 2487 mmol) were dissolved in a mixed solvent of N,N-dimethylformamide (DMF, 250 mL) and toluene (500 mL). Potassium carbonate (206.2 g, 1492 mmol) was added to this dark solution and the reaction mixture was stirred at 115 °C for 8 hours. Upon completion of the reaction, the reaction endpoint was confirmed by thin layer chromatography (TLC) monitoring. Insoluble material was removed by filtration followed by removal of solvent and excess 4-fluorobenzonitrile by distillation under reduced pressure. Ethyl acetate (800 mL), water (500 mL) and brine (40 mL) were added to the residue and phase separation was carried out at 65-70 °C. The aqueous phase was extracted with ethyl acetate (150 mL) at the same temperature and the combined organic phases were washed sequentially with mixed water (225 mL) and brine (180 mL) at 65-70 °C. The organic phase was concentrated under reduced pressure to give a brown solid product. The solid was purified by twice recrystallization in a mixture of hot ethyl acetate and toluene solvents to give 4-(4-bromo-3-formylphenoxy) benzyl cyanide (Compound A) (107.5 g) in 71% yield. High performance liquid chromatography (HPLC) analysis showed purity greater than 99%. Nuclear magnetic resonance hydrogen spectrum (1H-NMR, 200 MHz, CDCl3, δ ppm): 10.32 (1H, s); 7.62-7.72 (3H, m); 7.57-7.59 (1H, dd; J = 3 Hz, J = 1 Hz); 7.18-7.23 (1H, dd, J = 8 Hz, J = 3 Hz), 7.01-7.08 ( 2H, m). Nuclear magnetic resonance carbon spectrum (13C-NMR, 50 MHz, CDCl3, δ ppm): 190.69; 160.08; 155.13; 135.59; 134.89; 134.39; 126.86; 121.81; 120.28; 118.68; 118.33; 107.32. differential scanning calorimetry (DSC) Showed a heat absorption peak at 11.19°C.

1194-02-1
525 suppliers
$6.00/10g

2973-80-0
395 suppliers
$6.00/1g

906673-54-9
199 suppliers
inquiry
Yield:906673-54-9 76%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100; for 10 h;
Steps:
1.1 Step 1 Synthesis of Compound 3
Compound 1 (2.0 g, 16.5 mmol), Compound 2 (3.3 g, 16.5 mmol) and potassium carbonate were sequentially placed.(3.4 g, 24.8 mmol) was added to a solution of DMF (20 ml), and the mixture was stirred at 100 ° C for about 10 h, and analyzed by spotting until the starting material was completely reacted.The reaction solution was cooled to room temperature.The reaction was quenched with water (15 mL)EtOAcThe organic phase was washed twice with saturated sodium chloride. The organic phase was collected, dried over anhydrous sodium sulfate and evaporated.The concentrate was subjected to column separation (eluent: petroleum ether/ethyl acetate (v/v) = 10:1),The white solid product was obtained in 3.8 g, yield 76%.
References:
CN109206446,2019,A Location in patent:Paragraph 0116; 0118; 0119; 0120; 0121
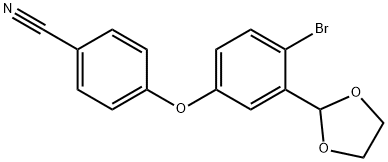
1217366-74-9
104 suppliers
inquiry

906673-54-9
199 suppliers
inquiry
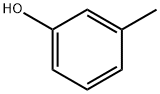
108-39-4
514 suppliers
$13.00/25g

906673-54-9
199 suppliers
inquiry
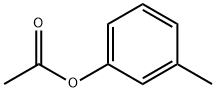
122-46-3
116 suppliers
$21.00/1g

906673-54-9
199 suppliers
inquiry

100-83-4
648 suppliers
$5.00/10g

906673-54-9
199 suppliers
inquiry