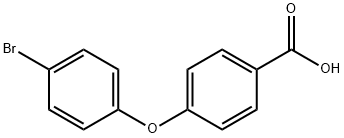
4-(4-BROMOPHENOXY)BENZOIC ACID 97 synthesis
- Product Name:4-(4-BROMOPHENOXY)BENZOIC ACID 97
- CAS Number:21120-68-3
- Molecular formula:C13H9BrO3
- Molecular Weight:293.11

21120-75-2
14 suppliers
$97.00/100mg

21120-68-3
33 suppliers
$54.00/1g
Yield:21120-68-3 99.1%
Reaction Conditions:
with lithium hydroxyde monohydrate;water monomer in tetrahydrofuran;methanol at 0 - 20; for 2 h;
Steps:
1.3 Step 3: 4-(4-bromophenoxy)benzoic acid (E).
To a solution of compound D (3.8 g, 12.4 mmol) in MeOH (20 mL), THF (10 mL) and water (10 mL) was added LiOH·H2O (2.6 g, 61.9 mmol) at 0 °C and the mixture was stirred at room temperature for 2 hours. The mixture was concentrated to 1/5 volume, diluted with water and washed with MTBE twice. The aqueous layer was acidified with 1 N aq. HCl to pH ~3 and extracted with DCM twice. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated to dryness under reduced pressure to give compound E (3.6 g, yield 99.1%) as a white solid, which was used directly in next step without further purification. LC/MS (ESI) (m/z): 291/293 (M-H)-.
References:
WO2022/66774,2022,A1 Location in patent:Page/Page column 239; 316-317
101-55-3
243 suppliers
$8.00/5g

21120-68-3
33 suppliers
$54.00/1g
201230-82-2
1 suppliers
inquiry
2050-47-7
257 suppliers
$6.00/5g
2215-89-6
287 suppliers
$6.00/10g

21120-68-3
33 suppliers
$54.00/1g

2050-47-7
257 suppliers
$6.00/5g

21120-68-3
33 suppliers
$54.00/1g
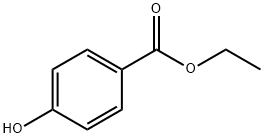
120-47-8
664 suppliers
$5.00/100mg

21120-68-3
33 suppliers
$54.00/1g