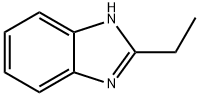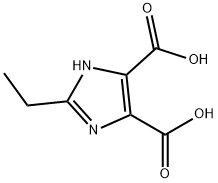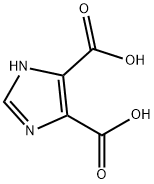
4,5-Imidazoledicarboxylic acid synthesis
- Product Name:4,5-Imidazoledicarboxylic acid
- CAS Number:570-22-9
- Molecular formula:C5H4N2O4
- Molecular Weight:156.1
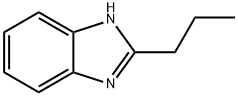
5465-29-2

570-22-9
General procedure: 800 ml of concentrated sulfuric acid (80% concentration) was slowly added to 200 ml of water to form a reaction system. To this system was added 50.0 g of 2-propylbenzimidazole, followed by the slow dropwise addition of 30 mL of hydrogen peroxide to 500 mL of the reaction mixture. The reaction is exothermic and causes reflux, and the rate of titration needs to be controlled to maintain the reaction solution at a boil. After completion of the reaction, stirring was continued for 3 hours. Subsequently, 1000 mL of water was added to the reaction mixture and cooled to room temperature. The mixture was stirred for 3 hours at 0 to 5°C to promote precipitation of solids. The solid was collected by filtration and washed sequentially with water and 5% aqueous sodium sulfite solution (100 mL). The resulting solid was purified by column chromatography using water: methanol = 2:1 as eluent to give the final imidazole-4,5-dicarboxylic acid 3-lg with a melting point of 267.3 °C (simultaneous decomposition).
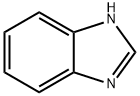
51-17-2
593 suppliers
$14.00/25g

570-22-9
306 suppliers
$10.00/5g
Yield:570-22-9 73%
Reaction Conditions:
with Iron(III) nitrate nonahydrate;tetrabutylammomium bromide;nitric acid in water at 80; for 8 h;
Steps:
18.1-18.3; 19.1-19.3; 22.1-22.3; 23.1-23.3; 24.1-24.3 Example 19
(1) Put 30 g of water into the reaction kettle, turn on the stirring, and sequentially add 0.06 g of Fe (NO3) 3.9H2O, 15 g of 96% sulfuric acid, 0.03 g of tetrabutylamine bromide, 2.5 g of benzimidazole, and raise the temperature;(2) When the reaction temperature reaches 80 ° C, 20 g of oxone is added in batches, and the reaction is incubated for 8 hours after the addition.(3) After the reaction is completed, the temperature is lowered to -10 to 10 ° C, and concentrated ammonia is slowly added dropwise under stirring to adjust the pH value to 0.6 to 1, and then stirring is continued for 1 hour for crystallization, suction filtration, and drying to obtain 4,5-imidazole di Carboxylic acid, the filtrate can be used for further product recovery, yield 73%.
References:
Changshu Institute of Technology;Yang Yang;Fu Renzhong;Wang Xin;Fang Zhengjiao;Lu Zhengyi;Zeng Xiaojun CN110818628, 2020, A Location in patent:Paragraph 0133-0140; 0149-0160
615-15-6
318 suppliers
$9.00/1g

570-22-9
306 suppliers
$10.00/5g
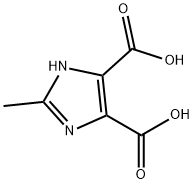
5313-35-9
35 suppliers
$109.00/50mg
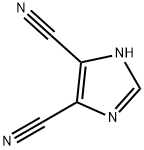
1122-28-7
430 suppliers
$6.00/5g

570-22-9
306 suppliers
$10.00/5g