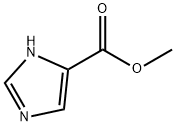
Methyl 4-imidazolecarboxylate synthesis
- Product Name:Methyl 4-imidazolecarboxylate
- CAS Number:17325-26-7
- Molecular formula:C5H6N2O2
- Molecular Weight:126.11

67-56-1
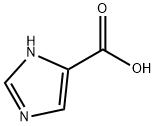
1072-84-0

17325-26-7
Step 1 Synthesis of methyl 1H-imidazole-4-carboxylate: 1H-imidazole-4-carboxylic acid (1.0 g, 8.9 mmol) was mixed with concentrated sulfuric acid (1 mL) in methanol (30 mL), stirred and heated to 0 °C. The reaction mixture was reacted under reflux conditions overnight. Upon completion of the reaction, the mixture was concentrated under reduced pressure and subsequently partitioned between cold water and ethyl acetate. The organic layer was separated and concentrated under reduced pressure to afford methyl 1H-imidazole-4-carboxylate (1 g, 89% yield).

67-56-1
790 suppliers
$7.29/5ml-f

1072-84-0
380 suppliers
$10.00/1g

17325-26-7
226 suppliers
$13.00/250mg
Yield:17325-26-7 99%
Reaction Conditions:
with thionyl chloride at 70; for 16 h;
Steps:
1 Step 1-Methyl 1H-imidazole-4-carboxylate
To a mixture of 1H-imidazole-4-carboxylic acid (5.00 g, 44.6 mmol, CAS1072-84-0) in MeOH (50 mL) was added SOCl2 (10.6 g, 89.2 mmol, 6.47 mL). The reaction mixture was stirred at 70° C. for 16 hours. On completion, the reaction mixture was concentrated in vacuo to give the title compound (5.60 g, 99% yield) as white solid. The residue was used to the next step directly without further purification. 1H NMR (400 MHz, DMSO-d6) δ 9.29-9.25 (m, 1H), 8.37 (s, 1H), 3.87 (s, 3H).
References:
Kymera Therapeutics, Inc.;Mainolfi, Nello;Ji, Nan;Kluge, Arthur F.;Weiss, Matthew M.;Zhang, Yi US2019/192668, 2019, A1 Location in patent:Paragraph 2699; 2700

67-56-1
790 suppliers
$7.29/5ml-f
3034-50-2
450 suppliers
$6.00/1g

17325-26-7
226 suppliers
$13.00/250mg

1072-84-0
380 suppliers
$10.00/1g

17325-26-7
226 suppliers
$13.00/250mg
31458-33-0
97 suppliers
$26.00/100mg
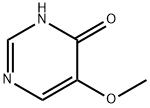
695-87-4
46 suppliers
$60.00/100mg

17325-26-7
226 suppliers
$13.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f

1072-84-0
380 suppliers
$10.00/1g

17325-26-7
226 suppliers
$13.00/250mg