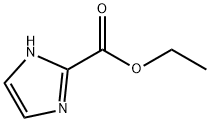
ETHYL IMIDAZOLE-2-CARBOXYLATE synthesis
- Product Name:ETHYL IMIDAZOLE-2-CARBOXYLATE
- CAS Number:33543-78-1
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14

64-17-5
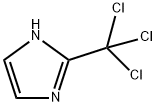
163769-73-1

33543-78-1
The above residue was dissolved in ethanol (300 mL). Concentrated sulfuric acid (98%, 30 mL, 522 mmol, 2.76 eq.) was added slowly and dropwise with stirring while controlling the reaction temperature to not exceed 25°C. The reaction mixture was heated and refluxed and stirred for 7 hours, followed by continued stirring at room temperature overnight. Upon completion of the reaction, ethanol was removed by distillation under reduced pressure. The resulting suspension was diluted with ice water (200 mL) and the pH was adjusted to 5-6 with concentrated ammonia, keeping the temperature below 5 °C during the process. The solid product was collected by filtration, washed with water (10 mL x 3) and dried under vacuum to give the first crude product (10 g). The filtrate was extracted with ethyl acetate (200 mL×2), the organic phases were combined, washed with saturated brine, dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure, combined with the first batch of crude product, and finally recrystallized in isopropyl ether to obtain ethyl 1H-imidazole-2-carboxylate (18 g, yield 64.3%).

288-32-4
1039 suppliers
$5.00/25g

124-38-9
134 suppliers
$214.00/14L

75-03-6
369 suppliers
$11.00/5g

33543-78-1
215 suppliers
$15.00/1/ G
Yield:33543-78-1 76 %
Reaction Conditions:
Stage #1: 1H-imidazole;carbon dioxidewith potassium tert-butylate;caesium carbonate in 1,2-dimethoxyethane at 120; under 76.0051 Torr;Sealed tube;
Stage #2: ethyl iodide in N,N-dimethyl-formamide at 50;regioselective reaction;Time;Temperature;Solvent;Reagent/catalyst;
Steps:
Procedure for carboxylation reaction
General procedure: To 10 mL sealed Schlenk tube ventilated with CO2 added substrate (0.50 mmol), t-Bu0K (0.60 mmol), Cs2CO3(0.60 mmol) and DMF (3.0 mL). The mixture was stirred for 18 h at 120 °C under 0.10 MPa carbon dioxideatmosphere. After the mixture was cooled to 50 °C, Iodoethane (3.0 equiv.) was added to the mixture. and the reactionmixture was stirred at 50 °C for 2 h. at ambient temperature, the product was extracted with CH2Cl2 (5×5 mL) andwashed with saturated NaCl aqueous solution (5×5 mL). The organic phase was dried by Na2SO4, then concentratedby distillation and purified by column chromatography.
References:
Zhang, Chengyan;Chen, Yanjiao;Wang, Yaya;Peng, Xinhua [Synthesis,2022] Location in patent:supporting information

64-17-5
825 suppliers
$10.00/50g

163769-73-1
9 suppliers
inquiry

33543-78-1
215 suppliers
$15.00/1/ G
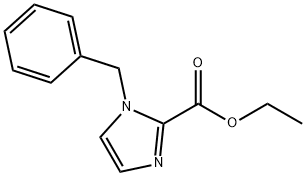
865998-45-4
35 suppliers
inquiry

33543-78-1
215 suppliers
$15.00/1/ G