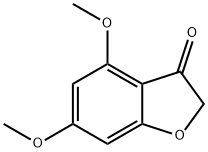
4,6-Dimethoxy-2,3-dihydrobenzofuran-3-one synthesis
- Product Name:4,6-Dimethoxy-2,3-dihydrobenzofuran-3-one
- CAS Number:4225-35-8
- Molecular formula:C10H10O4
- Molecular Weight:194.18
Yield:4225-35-8 97%
Reaction Conditions:
with potassium carbonate in 1,2-dimethoxyethane; for 5 h;Reflux;
Steps:
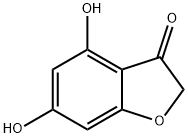
4.1.2 4,6-dimethoxybenzofuran-3(2H)-one (3)
To a solution of 4,6-dihydroxybenzofuran-3(2H)-one 2 (2.00 g, 12.0 mmol) in 1,2-dimethoxyethane (50 mL) were added potassium carbonate (3.70 g, 26.5 mmol) and dimethyl sulfate (2.50 mL, 26.5 mmol), and the mixture was refluxed for 5 h.
After cooling, solvents were removed under reduced pressure, water (40 mL) was added, and the mixture was extracted with CH2Cl2.
The combined organic layers were washed with water then brine, dried over anhydrous MgSO4, filtered off, and the filtrate was concentrated under reduced pressure.
The residue was purified by flash chromatography on silica gel (eluent cyclohexane/EtOAc 40:60) to afford 3 (2.25 g, 11.6 mmol, 97%) as an orange solid. m.p. 136 °C; 1H NMR (400 MHz, CDCl3) δ ppm 3.86 (s, 3H), 3.90 (s, 3H), 4.59 (s, 2H), 6.01 (d, J = 1.8 Hz, 1H), 6.15 (d, J = 1.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ ppm 56.0 (CH3), 56.1 (CH3), 75.5 (C), 88.9 (CH), 93.0 (CH), 104.8 (C), 158.8 (C), 169.8 (C), 177.1 (C), 195.0 (C).
References:
Meguellati, Amel;Ahmed-Belkacem, Abdelhakim;Nurisso, Alessandra;Yi, Wei;Brillet, Rozenn;Berqouch, Nawel;Chavoutier, Laura;Fortuné, Antoine;Pawlotsky, Jean-Michel;Boumendjel, Ahcène;Peuchmaur, Marine [European Journal of Medicinal Chemistry,2016,vol. 115,p. 217 - 229]
19728-23-5
2 suppliers
inquiry

4225-35-8
15 suppliers
inquiry