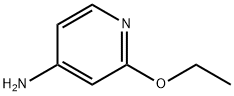
4-Amino-2-ethoxypyridine synthesis
- Product Name:4-Amino-2-ethoxypyridine
- CAS Number:89943-12-4
- Molecular formula:C7H10N2O
- Molecular Weight:138.17
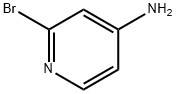
7598-35-8

89943-12-4
The general procedure for the synthesis of 2-ethoxy-4-aminopyridine from 4-amino-2-bromopyridine is as follows: 1. 2-Bromo-4-aminopyridine (4 g), solid sodium hydroxide (4 g) and ethanol (15 ml) were placed in a reactor and heated at 160°C for 6 hrs followed by cooling to room temperature. 2. Water was added to the reaction mixture and then the solvent was partially evaporated under reduced pressure. 3. The mixture was extracted using ether and the solvent was subsequently evaporated to give a clarified oily product of 2-ethoxy-4-aminopyridine (164 g, 76% yield). 4. The oily product slowly crystallized to form colorless needle-like crystals with a melting point of 88-89 °C. The product was extracted with ether and subsequently evaporated.

7598-35-8
361 suppliers
$6.00/250mg

89943-12-4
96 suppliers
$25.00/250mg
Yield: 76%
Reaction Conditions:
with sodium hydroxide in ethanol;water
Steps:
19.l f
l) 2-Ethoxy-4-aminopyridine (Den Hertog, Kolder and Combe, 1951) 2-Bromo-4-aminopyridine (4 g). solid sodium hydroxide (4 g) and ethanol (15 ml) were heated in a bomb at 160° C. for 6 hours and allowed to cool. Water was then added and the solvent partially removed under reduced pressure. The mixture was then extracted with ether and the solvent removed, leaving a clear oil of 2-Ethoxy-4-aminopyridine (164 g, 76%) which slowly crystallized into colourless needles, m.p. 88-89° C.
References:
Polychip Pharmaceuticals Pty Ltd;The University of Sydney US5929082, 1999, A
14432-12-3
544 suppliers
$5.00/5g

64-17-5
816 suppliers
$10.00/50g

89943-12-4
96 suppliers
$25.00/250mg

14432-12-3
544 suppliers
$5.00/5g

89943-12-4
96 suppliers
$25.00/250mg
26452-80-2
411 suppliers
$7.00/5g

89943-12-4
96 suppliers
$25.00/250mg
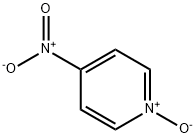
1124-33-0
337 suppliers
$10.00/1g

89943-12-4
96 suppliers
$25.00/250mg