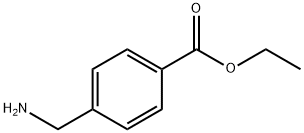
Ethyl 4-(aminomethyl)benzoate synthesis
- Product Name:Ethyl 4-(aminomethyl)benzoate
- CAS Number:366-84-7
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
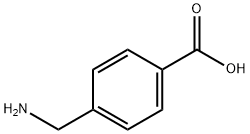
56-91-7

64-17-5

366-84-7
Example 1: Preparation of ethyl 4-(aminomethyl)benzoate Add 7.5 g (50 mmol) of 4-(aminomethyl)benzoic acid to a 250 mL three-necked round-bottomed flask. 100mL of anhydrous ethanol was added. Under the condition of ice bath, 20.8 g (175 mmol) of thionyl chloride was slowly added dropwise, and after completion of the dropwise addition, the reaction was stirred at room temperature for 30 min, followed by heating and refluxing for 4 hours. Upon completion of the reaction, the heating was stopped and the ethanol was removed by distillation under reduced pressure to give a white solid product. The resulting solid was dissolved in 150 mL of ethyl acetate, and the pH was adjusted to 7-8 by slowly adding 35% aqueous sodium hydroxide under the condition of an ice bath. the organic layer and the aqueous layer were separated, and the organic layer was washed with saturated saline three times, each time 30 mL. the organic layer was dried with anhydrous sodium sulfate, filtered, and concentrated to give a yellow solid product in 90% yield.
Yield: 90%
Reaction Conditions:
with thionyl chloride at 20; for 4.5 h;Cooling with ice;Reflux;
Steps:
1 Example 1 Preparation of p-aminomethylbenzoic acid ethyl ester
Example 1 Preparation of p-aminomethylbenzoic acid ethyl ester
In 250mL neck round bottom flask was added 7.5g (50mmol) p-aminomethylbenzoic acid. Added with 100mL anhydrous ethanol. In an ice bath, was slowly added thionyl chloride 20.8g (175mmol), after the addition was complete, stirring at room temperature after 30min, was heated at reflux for 4h. After stopping the heating, removal of the ethanol under reduced pressure to give a white solid. The solid was dissolved with 150mL ethyl acetate in an ice bath was slowly added 35% NaOH aqueous solution to pH 7-8. Still stratification, washed three times with saturated saline aqueous layer was removed, and the organic layer, each 30mL. Dried over anhydrous sodium sulfate, filtered, and concentrated to give a yellow solid, 90% yield.
References:
Tianjin Medical University;Dong, Weili;Wang, Runling;Yue, Hai;Wang, Shuqing;Deng, Zhirong CN104016942, 2016, B Location in patent:Paragraph 0090; 0091; 0092
1201-90-7
91 suppliers
$11.00/1g

366-84-7
53 suppliers
$22.00/100mg
26496-94-6
108 suppliers
$10.00/5g

366-84-7
53 suppliers
$22.00/100mg
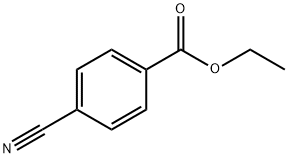
7153-22-2
159 suppliers
$8.00/1g

366-84-7
53 suppliers
$22.00/100mg

64-17-5
825 suppliers
$10.00/50g
2393-20-6
111 suppliers
$35.00/1g

366-84-7
53 suppliers
$22.00/100mg