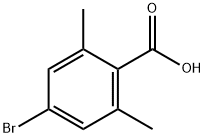
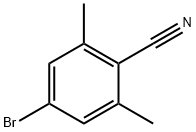
4-bromo-2,6-dimethylbenzoic acid synthesis
- Product Name:4-bromo-2,6-dimethylbenzoic acid
- CAS Number:74346-19-3
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07

124-38-9
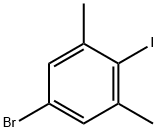
206559-43-5

74346-19-3
Example 2: Preparation of 4-bromo-2,6-dimethylbenzoic acid (B2) The title compound can be prepared according to Scheme B as follows. 5-Bromo-2-iodo-1,3-dimethylbenzene (B1) (1.0 mole equivalent) was dissolved in methyl tert-butyl ether (6.0 relative volume), and this solution was slowly added to a solution of isopropylmagnesium chloride (2.0 mole equivalents) in methyl tert-butyl ether (2.0 relative volume) that had been pre-cooled to < 0 °C. Carbon dioxide gas was passed through until the reaction was complete while maintaining low temperature conditions. Upon completion of the reaction, 2N aqueous hydrochloric acid (4 relative volume) was added to quench the reaction, the organic and aqueous phases were separated and the aqueous phase was discarded. The organic phase was extracted with 1M aqueous sodium hydroxide solution (6.5 relative volume) and subsequently washed with methyl tert-butyl ether (4 relative volume). The aqueous phase was acidified by addition of 2N aqueous hydrochloric acid (4.5 relative volume) to precipitate the title compound (B2). The precipitate was collected by filtration, washed sequentially with water and heptane and dried to give a white crystalline solid in 85% yield. 1H NMR (400 MHz, CDCl3) δH 2.28 (6H, s), 7.35 (2H, s), 13.30 (1H, s).

124-38-9
134 suppliers
$175.00/23402

206559-43-5
133 suppliers
$10.00/1g

74346-19-3
100 suppliers
$87.00/1g
Yield: 85%
Reaction Conditions:
Stage #1:carbon dioxide;5-bromo-2-iodo-1,3-dimethyl-benzene with isopropylmagnesium chloride in tert-butyl methyl ether at 0;Grignard Reaction;
Stage #2: with hydrogenchloride;water
Steps:
2
Example 2: Preparation of 4-bromo-2,6-dimethylbenzoic acid (B2)(B2)The title compound may be prepared according to Scheme B, as follows. A solution of 5- bromo-2-iodo-l,3-dimethyl-benzene (Bl) (1.0 mol eq) in methyl-tert-butyl ether (6.0 rel vols) may be added to iso-propylmagnesium chloride (2.0 mol eq) in methyl-tert-butyl ether (2.0 rel vols), maintaining < 0 °C. Carbon dioxide gas may be added until reaction is shown to be complete. 2N aqueous hydrochloric acid may be added (4 rel vols) to quench the reaction, the phases can be separated and the aqueous phase discarded. The product may be extracted into 1M aqueous sodium hydroxide solution (6.5 rel vols), then washed with methyl tert-butyl ether (4 rel vols). The title compound (B2) may be precipitated by the addition of 2N aq hydrochloric acid (4.5 rel vols) before being filtered, washed with water then heptane and dried to yield a white crystalline solid (85%).1H NMR (400 MHz, CDC13) δΗ 2.28 (6 H, s), 7.35 (2 H, s), 13.30 (1H, s)
References:
ASTRAZENECA AB;BOYD, Alistair;FIELDING, Mark Richard;FORD, James Gair;FRODSHAM, Lianne;GOLDEN, Michael D;LESLIE, Kevin William;MCKEEVER-ABBAS, Ben;TOMLIN, Paula WO2011/84098, 2011, A1 Location in patent:Page/Page column 6-7
110-05-4
276 suppliers
$22.00/100mL
68837-59-2
350 suppliers
$5.00/1g

74346-19-3
100 suppliers
$87.00/1g
5757-66-4
74 suppliers
inquiry

74346-19-3
100 suppliers
$87.00/1g

132949-41-8
5 suppliers
inquiry

74346-19-3
100 suppliers
$87.00/1g
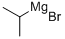
920-39-8
230 suppliers
$68.00/100ml

206559-43-5
133 suppliers
$10.00/1g

74346-19-3
100 suppliers
$87.00/1g