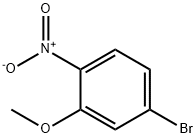
4-bromo-2-methoxy-1-nitrobenzene synthesis
- Product Name:4-bromo-2-methoxy-1-nitrobenzene
- CAS Number:103966-66-1
- Molecular formula:C7H6BrNO3
- Molecular Weight:232.03
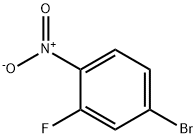
321-23-3

124-41-4

103966-66-1
GENERAL STEPS: To a solution of 4-bromo-2-fluoro-1-nitrobenzene (2.0 g, 9.09 mmol) in methanol (50 mL) was added a methanolic solution of 30% sodium methanolate (1.64 g, 9.09 mmol). The reaction mixture was stirred at room temperature overnight. After completion of the reaction, the reaction mixture was concentrated and the residue was dissolved in water (30 mL) and extracted with ethyl acetate (2 x 30 mL). The organic phases were combined, washed with water, separated, dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure to give 5-bromo-2-nitroanisole as a crystalline solid (2.1 g, 99% yield).1H-NMR (DMSO-d6): δ [ppm] 7.9 (d, 1H), 7.6 (s, 1H), 7.3 (d, 1H), 4.0 (s, 3H).

67-56-1
790 suppliers
$7.29/5ml-f

321-23-3
256 suppliers
$10.00/1g

124-41-4
731 suppliers
$12.00/25g

103966-66-1
130 suppliers
$7.00/100mg
Yield: 100%
Reaction Conditions:
at 60; for 3 h;Temperature;
Steps:
1.1; 2.1; 3.1 (1) Synthesis of compound III
Add 220g (1mol) of 4-bromo-2-fluoro-1-nitrotoluene, 1000ml of methanol to the reaction flask, add 108g (2mol) of sodium methoxide to 60°C, keep the temperature for 3 hours, and HPLC track the reaction until 4 The reaction of bromo-2-fluoro-1-nitrotoluene is complete; the reaction solution is concentrated, 1000 ml of water is added, and 1000 ml of ethyl acetate are added for layering, and the organic phase is concentrated to obtain 232 g of compound III. The molar yield: 100%.
References:
CN112300028, 2021, A Location in patent:Paragraph 0032; 0034-0035; 0052; 0054-0055; 0070; 0072-0073

321-23-3
256 suppliers
$10.00/1g

124-41-4
731 suppliers
$12.00/25g

103966-66-1
130 suppliers
$7.00/100mg
2398-37-0
580 suppliers
$8.00/10g
98447-30-4
87 suppliers
$10.00/100mg
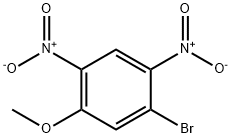
181995-71-1
7 suppliers
inquiry

103966-66-1
130 suppliers
$7.00/100mg

67-56-1
790 suppliers
$7.29/5ml-f

321-23-3
256 suppliers
$10.00/1g

103966-66-1
130 suppliers
$7.00/100mg

2398-37-0
580 suppliers
$8.00/10g

98447-30-4
87 suppliers
$10.00/100mg

103966-66-1
130 suppliers
$7.00/100mg