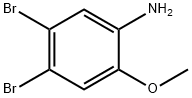
4-BROMO-2-METHOXY-PHENYLAMINE synthesis
- Product Name:4-BROMO-2-METHOXY-PHENYLAMINE
- CAS Number:59557-91-4
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
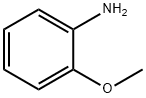
90-04-0

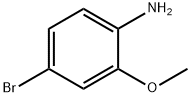
59557-91-4
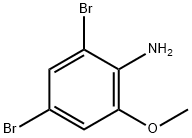
88149-47-7
General procedure for the synthesis of 2-methoxy-4-bromoaniline and 2,4-dibromo-6-methoxyaniline from o-methoxyaniline: o-methoxyaniline (470 mg, 3.60 mmol) was dissolved in dichloromethane (8 mL) and cooled to -10 °C. 2,4,4,6-tetrabromocyclohexanedione (1.56 g, 3.60 mmol) was added slowly under stirring, keeping the reaction temperature below -5 °C. The reaction mixture was gradually warmed to room temperature and stirring was continued overnight. Upon completion of the reaction, the reaction mixture was washed sequentially with 2M sodium hydroxide solution (10 mL) and water (15 mL), then dried over magnesium sulfate, filtered and concentrated to dryness. The residue was separated by silica gel column chromatography (eluent: dichloromethane) and first eluted to give 2,4-dibromo-6-methoxyaniline (90 mg, 9% yield) as a dark brown liquid. Its 1H NMR (400 MHz, CDCl3) data were as follows: δ 3.84 (s, 3H, OCH3), 4.20 (br s, 2H, NH2), 6.83 (d, J = 2.1 Hz, 1H, ArH), 7.19 (d, J = 2.1 Hz, 1H, ArH), which is in accordance with the literature reports. Continued elution gave 4-bromo-2-methoxyaniline (579 mg, 83% yield) as a brown solid with a melting point of 57-59 °C (literature values: 56.5-58 °C and 60-61 °C). Its 1H NMR (400 MHz, CDCl3) data were as follows: δ 3.83 (s, 3H, OCH3), 3.85 (br s, 2H, NH2), 6.60 (d, J = 7.8 Hz, 2H, ArH), and 6.88-6.90 (m, 2H, ArH), which is in accordance with the literature reports. Ref: J.-M. Chretien, F. Zammattio, E. Le Grognec, M. Paris, B. Cahingt, G. Montavon, and J.-P. Quintard, J. Org. Chem. 70 (2005), pp. 2870-2873.

90-04-0
377 suppliers
$5.00/5 g

59557-91-4
226 suppliers
$10.00/1g

88149-47-7
30 suppliers
$45.00/100mg
Yield:59557-91-4 83% ,88149-47-7 9%
Reaction Conditions:
with 2,4,4,6-tetrabromocyclohexadione in dichloromethane at -10 - 20;
Steps:
4.2.7. 4-Bromo-2-methoxyaniline
o-Anisidine (470 mg, 3.60 mmol) was dissolved in dichloromethane (8 mL) and cooled to -10 °C. 2,4,4,6-Tetrabromocyclohexadione (1.56 g, 3.60 mmol) was slowly added into the above stirred solution, while the temperature was kept below -5 °C. The reaction was allowed to warm to room temperature and stirring was continued overnight. The reaction mixture was washed with sodium hydroxide (10 mL, 2 M) and then water (15 mL), dried over magnesium sulfate, filtered and evaporated to dryness. The residue was chromatographed (silica gel, dichloromethane) to afford 2,4-dibromo-6-methoxyaniline (90 mg, 9%) as a dark brown liquid. 1H NMR (400 MHz, CDCl3) δ 3.84 (s, 3H, OCH3), 4.20 (br s, 2H, NH2), 6.83 (d, J 2.1 Hz, 1H, ArH), 7.19 (d, J 2.1 Hz, 1H, ArH). The spectral data are in agreement with those reported in the literature.65 Continued elution afforded 4-bromo-2-methoxyaniline (579 mg, 83%) as a brown solid: mp 57-59 °C (lit.66 56.5-58 °C; lit.65 60-61 °C). 1H NMR (400 MHz, CDCl3) δ 3.83 (s, 3H, OCH3), 3.85 (br s, 2H, NH2) 6.60 (d, J 7.8 Hz, 2H, ArH), 6.88-6.90 (m, 2H, ArH). The spectral data are in agreement with those reported in the literature.65 J.-M. Chrétien, F. Zammattio, E. Le Grognec, M. Paris, B. Cahingt, G. Montavon and J.-P. Quintard, J. Org. Chem. 70 (2005), pp. 2870-2873. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (25)65
References:
Malik, Qasim M.;Ijaz, Sadia;Craig, Donald C.;Try, Andrew C. [Tetrahedron,2011,vol. 67,# 32,p. 5798 - 5805] Location in patent:experimental part
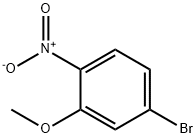
103966-66-1
131 suppliers
$7.00/100mg

59557-91-4
226 suppliers
$10.00/1g

90-04-0
377 suppliers
$5.00/5 g

59557-91-4
226 suppliers
$10.00/1g
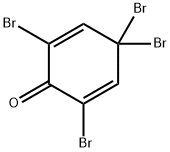
20244-61-5
123 suppliers
$22.39/1G

90-04-0
377 suppliers
$5.00/5 g

59557-91-4
226 suppliers
$10.00/1g