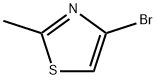
4-BROMO-2-METHYLTHIAZOLE synthesis
- Product Name:4-BROMO-2-METHYLTHIAZOLE
- CAS Number:298694-30-1
- Molecular formula:C4H4BrNS
- Molecular Weight:178.05
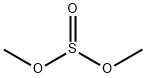
616-42-2

298694-30-1
General procedure for the synthesis of 2-methyl-4-bromothiazole from dimethyl sulfite: 2,4-dibromothiazole (2.4 g, 9.8 mmol) was dissolved in anhydrous THF (50 mL), and the resulting solution was stirred at -78 °C under argon protection. Slowly n-butyllithium (4.2 mL, 6 mmol, 2.5 M hexane solution) was added and stirring was continued for 1 hour. Subsequently, a solution of dimethyl sulfate (2.7 mL) in THF (5 mL) was added dropwise. The reaction mixture was stirred at -78 °C for 4 h before slowly warming to room temperature and continuing to stir overnight. Upon completion of the reaction, the reaction mixture was diluted with saturated aqueous sodium bicarbonate solution (50 mL). The aqueous layer was extracted with ether, the organic phases were combined, washed with brine, dried over magnesium sulfate and concentrated by rotary evaporation. Finally, purification by silica gel column chromatography afforded 2-methyl-4-bromothiazole as a yellow oil (0.956 g, 55% yield).

616-42-2
198 suppliers
$12.00/5g

298694-30-1
110 suppliers
$16.00/100mg
Yield:298694-30-1 55%
Reaction Conditions:
Stage #1:2,4-dibromo-1,3-thiazole with n-butyllithium in tetrahydrofuran;hexanes at -78; for 1 h;
Stage #2:dimethylsulfite in tetrahydrofuran;hexanes at -78 - 20;
Steps:
52
Example 52; 4-Bromo-2-methyl-thiazole (142); 2,4-Dibromothiazole (2.4 g, 9.8 mmol) was dissolved in abs. THF (50 ml) and the resulting solution was stirred under argon at -78 °C. A solution of BuLi (4.2 ml, 6 mmol, 2.5 M in hexanes) was added and the stirring was continued for Ih whereafter a solution of dimethylsulfate (2.7 ml) in THF (5 ml) was added dropwise. After stirring for 4h at -78 °C, the reaction mixture was warmed to room temperature and stirred EPO
References:
TIBOTEC PHARMACEUTICALS LTD.;MEDIVIR AB WO2007/14922, 2007, A1 Location in patent:Page/Page column 121-122
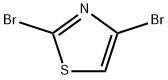
4175-77-3
326 suppliers
$6.00/1g

77-78-1
319 suppliers
$19.69/1ml

298694-30-1
110 suppliers
$16.00/100mg
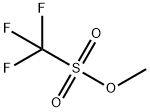
333-27-7
306 suppliers
$12.00/1g

298694-30-1
110 suppliers
$16.00/100mg