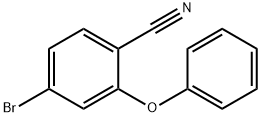
4-Bromo-2-phenoxybenzonitrile synthesis
- Product Name:4-Bromo-2-phenoxybenzonitrile
- CAS Number:875664-25-8
- Molecular formula:C13H8BrNO
- Molecular Weight:274.11
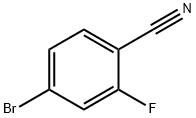
105942-08-3
369 suppliers
$7.00/5g

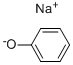
108-95-2
708 suppliers
$14.00/25g

875664-25-8
31 suppliers
inquiry
Yield:875664-25-8 91%
Reaction Conditions:
Stage #1: phenolwith potassium carbonate in N,N-dimethyl-formamide;toluene; for 4 h;Dean-Stark;Inert atmosphere;Reflux;
Stage #2: 4-bromo-6-fluorobenzonitrile in N,N-dimethyl-formamide;toluene; for 4 h;Reflux;Inert atmosphere;
Steps:
1 Synthesis of Compound 1
In a three-neck flask equipped with a Dean-Stark trap, phenol (12.35 g, 131.25 mmol), K2CO3 (34.55 g, 250 mmol), N,N-dimethylformamide (125 mL) and toluene (125 mL) were charged, and refluxed in a nitrogen atmosphere for 4 hours to perform dehydration until no further water was formed from the reaction system. Thereafter, 100 mL of toluene was removed with the Dean-Stark trap. (0176) After returning to room temperature, 4-bromo-2-fluorobenzonitrile (25.0 g, 125 mmol) was added thereto, and the mixture was refluxed in a nitrogen atmosphere for 4 hours. After completing the reaction, the solution was diluted by adding toluene (200 mL) thereto, and then filtered with Celite. The solution was rinsed twice with water with a separating funnel, dried over anhydrous magnesium sulfate, and filtered. The product was purified by silica gel chromatography (mobile phase: toluene/ethyl acetate=9/1), and a specimen deposited through concentration of the solution was rinsed with 200 mL of hexane under application of ultrasonic wave for 5 minutes, and then filtered. The specimen was dried in vacuum (50° C. for 4 hours) to provide a white solid matter (yield amount: 31.2 g, yield: 91%). The product was identified by 1H-NMR and ESI-MS. (0177) 1H NMR (500 MHz, CDCl3, δ): 6.97 (s, 1H), 7.11 (d, 2H), 7.25-7.31 (m, 2H), 7.42-7.48 (m, 2H), 7.51 (d, 1H) (0178) ESI-MS (m/z) (M+) calcd. 272.98, found 273.09.
References:
US9685615,2017,B2 Location in patent:Page/Page column 85-86
139-02-6
122 suppliers
$34.00/5g

105942-08-3
369 suppliers
$7.00/5g

875664-25-8
31 suppliers
inquiry
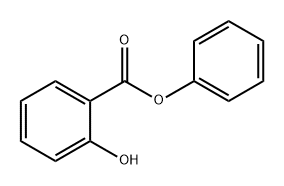
118-55-8
402 suppliers
$6.00/25g

875664-25-8
31 suppliers
inquiry
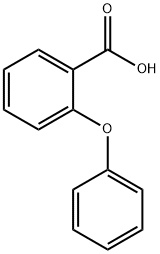
2243-42-7
147 suppliers
$8.00/1g

875664-25-8
31 suppliers
inquiry