
4-bromo-2-trifluoromethylphenylacetonitrile synthesis
- Product Name:4-bromo-2-trifluoromethylphenylacetonitrile
- CAS Number:877131-92-5
- Molecular formula:C9H5BrF3N
- Molecular Weight:264.04
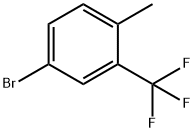
86845-27-4
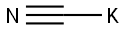
151-50-8

877131-92-5
The general procedure for the synthesis of 4-bromo-2-trifluoromethylphenylacetonitrile from 5-bromo-2-methyltrifluorotoluene and potassium cyanide was as follows: 4-methyl-3-trifluorobromobenzene (33.26 g, 104.261 mmol), potassium cyanide (20.43 g, 313.67 mmol) and tetrabutylammonium bromide (3.37 g, 10.45 mmol) were reacted in a methylene chloride/water (1:1, 300 mL) in a solvent mixture of dichloromethane/water, and the reaction was stirred at room temperature for 4 hours. After the reaction was completed, the organic layer and the aqueous layer were separated, and the organic layer was washed with water (100 mL), 1N hydrochloric acid (100 mL), and saturated saline, and dried over anhydrous sodium sulfate. The organic layer was concentrated under reduced pressure to obtain a brown oily crude product. The crude product was purified by fast column chromatography (eluent: 0% to 20% hexane solution of ethyl acetate) to afford the target product 4-bromo-2-trifluoromethylphenylacetonitrile (14.9 g, 54% yield) in the form of a colorless transparent oil. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 3.91 (s, 2H), 7.57 (d, J = 8.3 Hz, 1H), 7.75 (dd, J = 8.3, 2Hz, 1H), 7.84 (d, J = 2Hz, 1H).
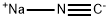
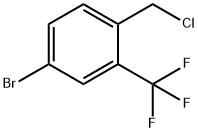
773837-37-9
0 suppliers
inquiry
1214350-42-1
7 suppliers
inquiry

877131-92-5
40 suppliers
$35.00/250mg
Yield:877131-92-5 92%
Reaction Conditions:
in water;acetonitrile at 80;Inert atmosphere;
Steps:
A.2.70.3 A.2.70.3. 2-(4-Bromo-2-(trifluoromethyl)phenyl)acetonitrile
A solution of 4-bromo-1-(chloromethyl)-2-(trifluoromethyl)benzene (2.707 g, 9.81 mmol) in MeCN (32 mL) and water (4 mL) is treated with sodium cyanide (0.651 g, 12.80 mmol) and the mixture is heated to 80°C, under nitrogen, overnight. The RM is allowed to cool to RT and is diluted with water. Acetonitrile is removedunder reduced pressure and the mixture is extracted twice with DCM. The combined organic layers are dried over anh. MgSO4, filtered and concentrated under reduced pressure. Purification by FC (from heptane to heptane/EtOAc = 7/3) affords 2-(4-bromo-2-(trifluoromethyl)phenyl)acetonitrile as a light yellow solid (2.385 g, 92%). LC-MS B: tR = 0.95 mm; no ionization.
References:
WO2018/210988,2018,A1 Location in patent:Page/Page column 207
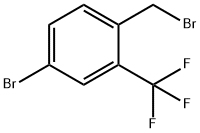
335013-18-8
67 suppliers
$19.00/100mg
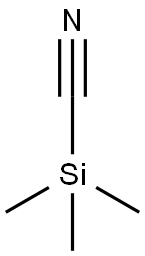
7677-24-9
394 suppliers
$19.00/5g

877131-92-5
40 suppliers
$35.00/250mg

86845-27-4
122 suppliers
$11.00/1g
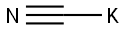
151-50-8
0 suppliers
$47.79/100g

877131-92-5
40 suppliers
$35.00/250mg

335013-18-8
67 suppliers
$19.00/100mg
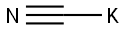
151-50-8
0 suppliers
$47.79/100g

877131-92-5
40 suppliers
$35.00/250mg
320-31-0
160 suppliers
$20.00/1g

877131-92-5
40 suppliers
$35.00/250mg