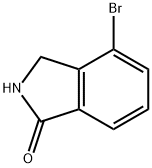
4-bromoisoindolin-1-one synthesis
- Product Name:4-bromoisoindolin-1-one
- CAS Number:337536-15-9
- Molecular formula:C8H6BrNO
- Molecular Weight:212.04
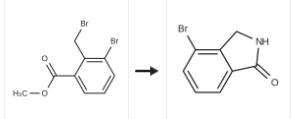
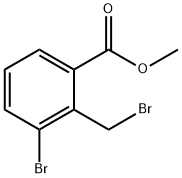
To a solution of the 3-bromo-2-bromomethyl-benzoic acid methyl ester (2.74 g, 8.88 [MMOL)] in tetrahydrofuran (70 [ML)] at [0°C] was added 30 percent aq. ammonia (10 ml) and the mixture stirred at room temperature under nitrogen for 18 hours. The solvent was removed by evaporation under reduced pressure. The white residue was partitioned between ethyl acetate (50 mi) and 2M citric acid (50 [ML).] The ethyl acetate was dried magnesium sulfate, filtered and solvent removed by evaporation under reduced pressure. The orange oil was dissolved in minimum [DICHLOROMETHANE] and purified by flash chromatography on silica gel eluting with a solvent gradient of [DICHOROMETHANE/METHANOL] (9: 1) to give the title compound (1.5 g, 80 percent) as a white solid.
337536-14-8
130 suppliers
$25.00/100mg

337536-15-9
149 suppliers
$34.82/1g
Yield:337536-15-9 99%
Reaction Conditions:
with ammonia in tetrahydrofuran;methanol at 0 - 20; for 7.5 h;
Steps:
3-bromo-2-methylbenzoic acid (6.13 g, 28.5 mmol) was suspended in MeOH (52 ml) and concentrated H2SO4 (10.0 ml) was added via syringe over 4 minutes at room temperature. The reaction was heated to 90 C, stirred for 4 hours, cooled in an ice water bath, and then quenched with saturated NaHCO3 (250 ml). The reaction was extracted with EtOAc (3 x 50 ml), and the organic layers were combined, dried over MgSO4, filtered, and concentrated to give methyl 3-bromo-2-methylbenzoate (6.43 g, 98%).Methyl 3-bromo-2-methylbenzoate (7.45 g, 32.5 mmol) was dissolved in CCl4 (94 ml) and N- bromosuccinimide (6.67 g, 37.5 mmol) and benzoyl peroxide (0.38 g, 1.6 mmol) were added. The reaction was heated to 75 C - 85 C, stirred for 3 hours and 45 minutes, cooled to room temperature, and filtered. The filtrate was concentrated and purified on SiO2 (Biotage instrument; 0% -> 20% EtOAc / hexanes) to give methyl 3-bromo-2-(bromomethyl)benzoate (10.07 g, 100%).Methyl 3-bromo-2-(bromomethyl)benzoate (10.30 g, 33.44 mmol) was dissolved in THF (93 ml) and cooled in an ice water bath. Then, ΝH3 (60 ml, ~ 7N in MeOH) was added via syringe over 4.5 minutes. The reaction was warmed to room temperature, stirred for 7.5 hours, and then diluted with water. The aqueous phase was extracted repeatedly with CH2Cl2 and EtOAc. The organic extracts were combined, dried over sodium sulfate, filtered, and concentrated to give title compound (6.99 g, EPO
References:
WO2007/5668,2007,A2 Location in patent:Page/Page column 36-37
![2-Pyridinecarboxamide, N-[(2-bromophenyl)methyl]-](/CAS/20210305/GIF/1291713-01-3.gif)
1291713-01-3
0 suppliers
inquiry
1972-28-7
398 suppliers
$15.00/5g

337536-15-9
149 suppliers
$34.82/1g
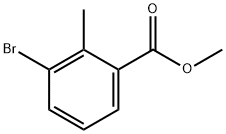
99548-54-6
171 suppliers
$5.00/1g

337536-15-9
149 suppliers
$34.82/1g
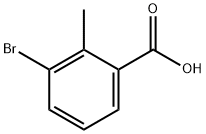
76006-33-2
321 suppliers
$10.00/5g

337536-15-9
149 suppliers
$34.82/1g
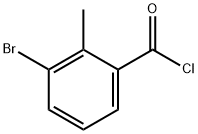
21900-48-1
35 suppliers
$60.00/100mg

337536-15-9
149 suppliers
$34.82/1g