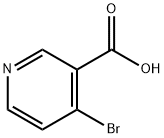
4-Bromonicotinic acid synthesis
- Product Name:4-Bromonicotinic acid
- CAS Number:15366-62-8
- Molecular formula:C6H4BrNO2
- Molecular Weight:202.01

124-38-9
134 suppliers
$175.00/23402
19524-06-2
485 suppliers
$6.00/5g

15366-62-8
167 suppliers
$14.00/250mg
Yield:-
Reaction Conditions:
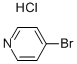
Stage #1:4-bromopyridine hydrochloride with n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 2 h;Inert atmosphere;
Stage #2:carbon dioxide in tetrahydrofuran at 20;
Stage #3: with hydrogenchloride;water; pH=3 - 4
Steps:
3
Preparation 3 3'-Hydroxymethyl-3,4,5,6-tetrahydro-2H-[4,4]bipyridinyl-1-carboxylic Acid t-Butyl Ester Butyllithium (82.0 mL, 205.6 mmol, 2.0 eq.) was added dropwise to a solution of diisopropylamine (20.8 g, 205.6 mmol, 2.0 eq.) in THF (100 mL) at -78° C., under nitrogen. The resulting solution was stirred for 1 hour at approximately -60 to -50° C. The resulting solution was added dropwise to a solution of 4-bromopyridine hydrochloride (20.0 g, 102.9 mmol, 1.0 eq.) in THF (100 mL) at -78° C. The resulting solution was stirred for 2 hours. Carbon dioxide gas was introduced into the reaction vessel for 30 minutes. The resulting solution was stirred overnight at room temperature. The reaction was then quenched with water (100 mL). The resulting solution was extracted with EtOAc (3*200 mL) and the aqueous layers were combined. The pH of the solution was adjusted to 3-4 with 1N HCl to yield 4-bromonicotinic acid (16.8 g) as a white solid. ES m/z: [M+H]+ 202. H-NMR: (300 MHz, DMSO, ppm): 8.88 (1H, s), 8.53 (1H, d, J=5.4 Hz), 7.84 (1H, d, J=5.4 Hz).
References:
Stangeland, Eric L.;Patterson, Lori Jean;Zipfel, Sheila US2011/230495, 2011, A1 Location in patent:Page/Page column 20-21