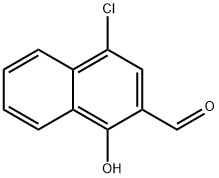
4-chloro-1-hydroxynaphthalene-2-carbaldehyde synthesis
- Product Name:4-chloro-1-hydroxynaphthalene-2-carbaldehyde
- CAS Number:58132-06-2
- Molecular formula:C11H7ClO2
- Molecular Weight:206.63
Yield:58132-06-2 403 mg
Reaction Conditions:
in trifluoroacetic acid at 80; for 1 h;Inert atmosphere;
Steps:
67-(a) Prodution of 4-chloro-1-hydroxy-2-naphthoaldehyde
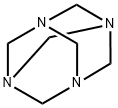
To a solution of 4-chloronaphthalen-1-ol (800 mg) in trifluoroacetic acid (5 mL) was added hexamethylenetetramine (879 mg) under argon gas flow with stirring at room temperature, and the resulting mixture was stirred at 80°C for 1 hour. After the reaction was completed, the reaction solution was concentrated under reduced pressure, and a saturated aqueous solution of sodium hydrogen carbonate was added thereto. The resulting mixed solution was subjected to extraction with ethyl acetate. The resulting organic layer was washed sequentially with a saturated aqueous solution of sodium hydrogen carbonate and saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The resulting residues were purified by a silica gel column (eluent: hexane : ethyl acetate) to give the title compound (403 mg) as yellow solids. Mass spectrum (ESI, m/z): 207 [M+H]+
References:
EP3978073,2022,A1 Location in patent:Paragraph 1463-1465
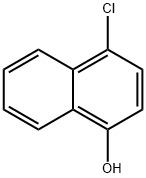
604-44-4
204 suppliers
$17.46/5G

58132-06-2
7 suppliers
inquiry
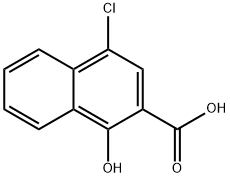
5409-15-4
32 suppliers
$85.00/250mg

58132-06-2
7 suppliers
inquiry
86-48-6
341 suppliers
$5.00/25g

58132-06-2
7 suppliers
inquiry