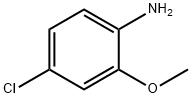
4-CHLORO-2-ANISIDINE HYDROCHLORIDE synthesis
- Product Name:4-CHLORO-2-ANISIDINE HYDROCHLORIDE
- CAS Number:93-50-5
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
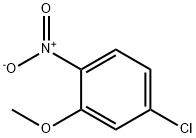
6627-53-8

93-50-5
General procedure: Make a suspension of iron powder (5.0 eq.), ammonium chloride (0.65 eq.) and distilled water and reflux for 15 minutes. 2-Nitro-5-chloroanisole (1.0 eq.) was added slowly and the reaction mixture continued to be stirred under reflux conditions. The progress of the reaction was monitored by thin layer chromatography (TLC) and when the reaction was complete, the reaction mixture was neutralized by slow dropwise addition of 5% aqueous sodium bicarbonate, followed by filtration through diatomaceous earth. The filtrate was extracted three times with ethyl acetate. The organic phases were combined and washed once each with saturated saline and 5% aqueous hydrochloric acid, in that order. The aqueous phases were combined, the pH was adjusted to neutral with 20% aqueous sodium hydroxide, and then extracted three times with ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The product can be purified by mixed solvent column chromatography with ethyl acetate/hexane or used directly as a crude product. Product purity was confirmed by 1H-NMR. Example 1: The crude 4-chloro-2-methoxyaniline was obtained as a dark purple oil (1.15 g, 91% yield) from the reaction of iron powder (2.23 g, 40 mmol), ammonium chloride (278 mg, 5.2 mmol), water (48 mL), and 5-chloro-2-nitroanisole (1.5 g, 8.0 mmol) for 1.5 h. The reaction was carried out by 1H NMR ( 300MHz, CDCl3): δ 6.78 (m, 2H), 6.65 (d, 1H), 3.86 (s, 3H). Example 2: 4-Chloro-2-methoxyaniline crude was obtained as a dark purple oil (2.35 g, 93% yield) after reacting for 2 hours with iron powder (4.47 g, 80.0 mmol), ammonium chloride (556 mg, 10.4 mmol), water (80 mL) and 5-chloro-2-nitroanisole (3.0 g, 16.0 mmol). No 1H-NMR analysis was performed.

67-56-1
790 suppliers
$7.29/5ml-f
100-00-5
55 suppliers
$14.00/25g

93-50-5
143 suppliers
$21.00/250mg
Yield: 28%
Reaction Conditions:
with fac-tris(2-phenylpyridinato-N,C2')iridium(III);methanesulfonic acid;triphenylphosphine in acetonitrile at 55; for 24 h;Irradiation;Inert atmosphere;
Steps:
8 Examples 1-16
General procedure: Step 1: Add the nitro compound (see Table 1 for specific substances) and methanol into the reaction vessel, and add the reducing agent (with(See Table 1 for specific substances), photocatalyst (see Table 1 for specific substances), water (see Table 1 for specific materials), organic acids (see Table 1 for specific substances)1) Add the organic solvent (see Table 1 for specific substances) into the reaction vessel.
References:
CN112812027, 2021, A Location in patent:Paragraph 0027; 0030-0032; 0041-0042

6627-53-8
165 suppliers
$10.00/1g

93-50-5
143 suppliers
$21.00/250mg
90-04-0
377 suppliers
$5.00/5 g

93-50-5
143 suppliers
$21.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

93-50-5
143 suppliers
$21.00/250mg

1092349-89-7
31 suppliers
$144.00/5g

93-50-5
143 suppliers
$21.00/250mg