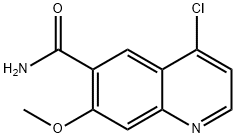
4-chloro-7-Methoxyquinoline-6-carboxaMide synthesis
- Product Name:4-chloro-7-Methoxyquinoline-6-carboxaMide
- CAS Number:417721-36-9
- Molecular formula:C11H9ClN2O2
- Molecular Weight:236.65
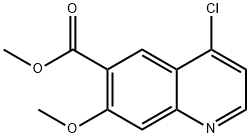
205448-66-4

417721-36-9
General procedure for the synthesis of 4-chloro-7-methoxyquinoline-6-amide from methyl 4-chloro-7-methoxyquinoline-6-carboxylate: 4.1) Ammonia (200 mL, 5.2 mol) was added to a 500 mL three-necked flask, followed by the addition of Compound IV (10 g, 39.8 mmol) obtained from Step 3.3) to obtain Mixture H; 4.2) Mixture H was was reacted at 60 °C for 4 h. After completion of the reaction, the mixture was cooled to room temperature to obtain mixture I. 4.3) Three extractions were performed by adding dichloromethane to mixture I, resulting in a light yellow solid compound V in 85.17% yield.
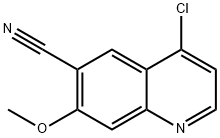
398487-31-5
21 suppliers
inquiry

417721-36-9
356 suppliers
$8.00/250mg
Yield:417721-36-9 91%
Reaction Conditions:
with dihydrogen peroxide;potassium carbonate in dimethyl sulfoxide at 5 - 20;
Steps:
1.3; 2.3; 3.3
(3) Put 500g of Intermediate II into a 5L reaction flask, add 1.5L DMSO and 105g potassium carbonate, The molar ratio of intermediate II to potassium carbonate is 3:1, the temperature is controlled at 5-10°C, and 246mL hydrogen peroxide (30%) is added dropwise. The molar ratio of hydrogen peroxide to Intermediate II is 1.05:1. After the dropwise addition is completed, return to room temperature for reaction. After the reaction is completed, the reaction solution is added to ice water, filtered, washed with water, and dried to obtain 490 g of 4-chloro-7-methoxyquinoline-6-carboxamide with a yield of 91%.
References:
CN112110856,2020,A Location in patent:Paragraph 0006; 0018; 0020; 0022-0023; 0025

205448-66-4
231 suppliers
$9.00/250mg

417721-36-9
356 suppliers
$8.00/250mg
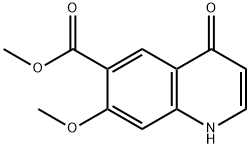
205448-65-3
179 suppliers
$13.00/100mg

417721-36-9
356 suppliers
$8.00/250mg
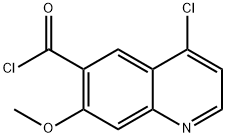
417721-35-8
5 suppliers
inquiry

417721-36-9
356 suppliers
$8.00/250mg
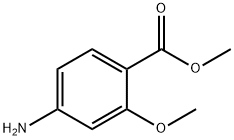
27492-84-8
314 suppliers
$6.00/5g

417721-36-9
356 suppliers
$8.00/250mg