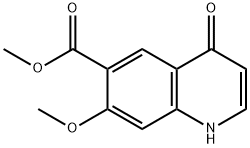
Methyl 7-Methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylate synthesis
- Product Name:Methyl 7-Methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylate
- CAS Number:205448-65-3
- Molecular formula:C12H11NO4
- Molecular Weight:233.22
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2

205448-65-3
General procedure for the synthesis of methyl 7-methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylate from methyl 4-((2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-yl)methylamino)-2-methoxybenzoate: 5-((3-methoxy-4-methoxycarbonyloxyanilino)methylene-2,2-dimethyl-1,3-dioxane-4, 6-dione (10 g, 29.8 mmol) was suspended in DOWTHERM A (125 ml) and heated to 180-190 °C within 30 min. The raw material dissolved at 100°C and carbon dioxide began to be released at about 180°C. Heating was continued for 30 minutes and then stopped. As the reaction mixture cooled, the product gradually precipitated. When the temperature drops to 40°C, ether is added and the mixture is stirred for 30 minutes. The solid product was collected by filtration, washed with ether and dried under vacuum to afford 7-methoxy-6-methoxycarbonyl-1,4-dihydroquinolin-4-one (5.56 g, 80% yield). The structure of the product was confirmed by 1H NMR spectroscopy: (DMSO-d6) δ 3.80 (s, 3H); 3.85 (s, 3H); 5.95 (d, 1H); 7.00 (s, 1H); 7.85 (d, 1H); 8.40 (s, 1H); 11.6 (br s, 1H); MS-ESI: 234 [MH]+.
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg

205448-65-3
180 suppliers
$13.00/100mg
Yield:205448-65-3 80.7%
Reaction Conditions:
in diphenylether;biphenyl at 170 - 185; for 0.75 h;Inert atmosphere;
Steps:
3
45 g (292 mmol) of biphenyl was weighed into a three-necked flask, 150 mL of diphenyl ether was added, the solvent was heated to 180 ° C under nitrogen atmosphere, and 18 g (53.7 mmol) of compound 4 was rapidly added under a nitrogen atmosphere, and a large amount of gas was released. The temperature was maintained at 170 to 185 ° C, and the reaction was continued for 45 minutes to stop the heating. Cooled to room temperature, a large amount of yellow solid precipitated, added petroleum ether, filtered, filter cakeThe crude product was washed well with diethyl ether.The crude product was purified by petrol-ethyl acetate (5:2 by volume), suction filtered and dried.A yellow solid (5) of 10.11 g was obtained in a yield of 80.7%.
References:
Nanjing Tian Yue Xing Biological Co., Ltd.;Wu Xueping;Chen Yao CN109456267, 2019, A Location in patent:Paragraph 0026; 0027
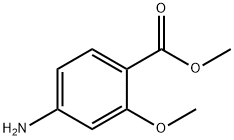
27492-84-8
314 suppliers
$6.00/5g

205448-65-3
180 suppliers
$13.00/100mg
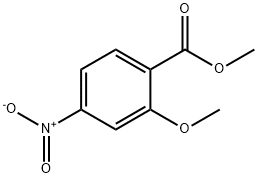
39106-79-1
37 suppliers
inquiry

205448-65-3
180 suppliers
$13.00/100mg
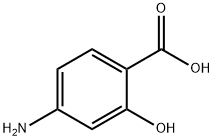
65-49-6
508 suppliers
$6.00/25g

205448-65-3
180 suppliers
$13.00/100mg