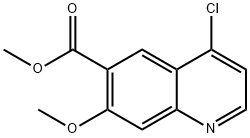
Methyl 4-chloro-7-Methoxyquinoline-6-carboxylate synthesis
- Product Name:Methyl 4-chloro-7-Methoxyquinoline-6-carboxylate
- CAS Number:205448-66-4
- Molecular formula:C12H10ClNO3
- Molecular Weight:251.67
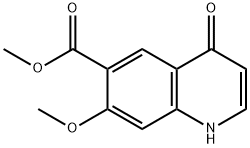
205448-65-3

205448-66-4
Methyl 7-methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylate (3.00 g, 12.86 mmol) was added to thionyl chloride (30.00 mL). N,N-dimethylformamide (93.99 mg, 1.29 mmol) was then added to the reaction system. The reaction solution was heated to an external temperature of 90 °C under nitrogen protection and reacted under reflux conditions for 1 hour. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the aqueous phase was combined and concentrated to dryness. The residue was dissolved in ice water (50 mL) and extracted with ethyl acetate (20 mL x 2). The aqueous phase was then extracted with dichloromethane (30 mL x 5). The dichloromethane phases were combined, washed with sodium chloride solution (20 mL × 2), dried over anhydrous sodium sulfate, and subsequently concentrated under reduced pressure by pumping to afford methyl 4-chloro-7-methoxyquinoline-6-carboxylate (2.60 g, 9.81 mmol) in 76.32% yield and 95% purity as a mixed solid. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 3.87 (s, 3H), 3.98 (s, 3H), 7.60 (s, 1H), 7.66 (d, J = 4.77 Hz, 1H), 8.41 (s, 1H), 8.83 (d, J = 4.77 Hz, 1H).

205448-65-3
181 suppliers
$13.00/100mg

205448-66-4
232 suppliers
$9.00/250mg
Yield:205448-66-4 94.53%
Reaction Conditions:
with trichlorophosphate in toluene at 25 - 95;
Steps:
1 Example 1: Preparation of Methyl 4-chloro-7-methoxyguinoline-6-carboxylate.
10.0 g of methyl 7-methoxy-4-oxo-1, 4-dthydroquinoline-6-carboxylate, 90 mL of toluene and 8.2 g of phosphoryl chloride were taken in a flask at 25-3OoC and heated to 90-95oC for 3-4 hours. The reaction was cooled to 25-3OoC and 100 mL of demineralized water was added to the above reaction mass. The organic layer was washed with 50 mL of demineralized water and to this 41 mL of 20% sodium hydroxide solution was added. The resulting compound was filtered, washed and dried under reduced pressure to obtain the title compound.Yield: 94.53 %; HPLC purity: 99.61%
References:
DR. REDDY'S LABORATORIES LIMITED;ARKALA, Anil Kumar Reddy;BHALERAO, Dinesh;MAHAPATRA, Tridib;MOVVA, Venkateswarlu;JINNA, Rajendar Reddy;ELATI, Raviram Chandrasekhar WO2019/92625, 2019, A1 Location in patent:Page/Page column 8; 9

205448-65-3
181 suppliers
$13.00/100mg

75-09-2
1263 suppliers
$10.00/25 mL

205448-66-4
232 suppliers
$9.00/250mg
150-75-4
82 suppliers
$78.00/250mg

205448-66-4
232 suppliers
$9.00/250mg
27492-84-8
315 suppliers
$6.00/5g

205448-66-4
232 suppliers
$9.00/250mg
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
80 suppliers
$25.00/250mg

205448-66-4
232 suppliers
$9.00/250mg