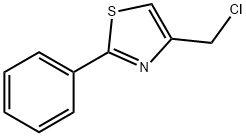
4-(Chloromethyl)-2-phenyl-1,3-thiazole synthesis
- Product Name:4-(Chloromethyl)-2-phenyl-1,3-thiazole
- CAS Number:4771-31-7
- Molecular formula:C10H8ClNS
- Molecular Weight:209.7
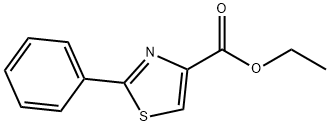
59937-01-8
70 suppliers
$43.00/250mg

4771-31-7
82 suppliers
$45.00/100mg
Yield: 89%
Reaction Conditions:
Stage #1:2-phenylthiazole-4-carboxylic acid ethyl ester with methanol;sodium tetrahydroborate in tetrahydrofuranReflux;
Stage #2: with thionyl chloride;N,N-dimethyl-formamide in dichloromethane for 4 h;
Steps:
4-(Chloromethyl)-2-phenylthiazole (32a).
To a stirred solution of compound 31a (1 g, 4.29 mmol) in a mixture of tetrahydrofuran (12 mL) and methanol (0.6 mL), sodium borohydride (0.48 g, 12.86 mmol) was added and the reaction mixture was transferred to an oil bath and heated to reflux. After the reaction was completed, the reaction solution was cooled to the ambient temperature and slowly added into ice water (20 mL). Ethyl acetate (40 mL) was added and the organic phase was separated and concentrated. The residue (0.9 g) was directly for the next reaction without purification. Dichloromethane (30 mL) and 5 drops of DMF were added to the residue and stirred at room temperature, followed by slow drops of sulfoxide chloride (2.0 ml, 28.24 mmol) and the reaction was carried out for 4 hours at room temperature. After the reaction was completed, the crude product was purified by column chromatography (petroleum ether/ethyl acetate = 30/1 ~ 15/1) to give compound 32a (0.8 g, 89% yield) as a white powdered solid.
References:
Fang, Lincheng;Hu, Zhaoxue;Yang, Yifei;Chen, Pan;Zhou, Jinpei;Zhang, Huibin [Bioorganic and Medicinal Chemistry,2021,vol. 39,art. no. 116133] Location in patent:supporting information
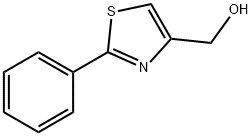
23780-13-4
75 suppliers
$27.00/100mg

4771-31-7
82 suppliers
$45.00/100mg
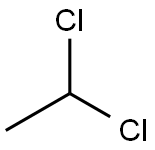
75-34-3
107 suppliers
$22.69/48602
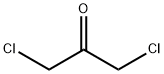
534-07-6
0 suppliers
$10.00/1g
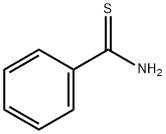
2227-79-4
237 suppliers
$6.00/5g

4771-31-7
82 suppliers
$45.00/100mg

2227-79-4
237 suppliers
$6.00/5g

4771-31-7
82 suppliers
$45.00/100mg