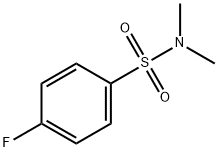
4-Fluoro-N,N-dimethylbenzenesulfonamide synthesis
- Product Name:4-Fluoro-N,N-dimethylbenzenesulfonamide
- CAS Number:383-31-3
- Molecular formula:C8H10FNO2S
- Molecular Weight:203.23
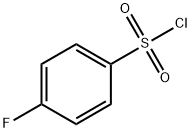
349-88-2

124-40-3

383-31-3
GENERAL STEPS: A mixture of 4-fluorobenzenesulfonyl chloride (2.0 g, 10.3 mmol), dimethylamine (2.0 M in methanol, 11.0 mL, 22.0 mmol), and dichloromethane (11.0 mL) was stirred at 0 °C, followed by continued stirring at room temperature for 5 min. Dichloromethane (30 mL) was added to dilute the reaction mixture. The organic phase was washed sequentially with 1 M hydrochloric acid (30 mL) and saturated brine (30 mL), dried over anhydrous magnesium sulfate and filtered. The solvent was removed by concentration under reduced pressure to give 4-fluoro-N,N-dimethylbenzenesulfonamide as a white solid (2.12 g, quantitative yield). A mixture of 4-fluoro-N,N-dimethylbenzenesulfonamide (1.50 g, 7.39 mmol), sodium thiosulfate (2.08 g, 29.7 mmol), and N,N-dimethylformamide (9.0 mL) was placed in a sealed tube and the reaction was stirred for 16 h at 170 °C. The reaction was completed by cooling to room temperature. After completion of the reaction, it was cooled to room temperature and 1 M sodium hydroxide solution (40 mL) was added. The aqueous phase was washed with ether (2 x 40 mL) and subsequently acidified with 2M hydrochloric acid to pH < 2 and extracted with ether (3 x 40 mL). The organic phases were combined, dried over anhydrous magnesium sulfate and filtered. The solvent was removed by concentration under reduced pressure to afford 4-mercapto-N,N-dimethylbenzenesulfonamide as an orange oil (503 mg, 31% yield).1H NMR (500 MHz, CDCl3) δ 7.67-7.58 (m, 2H), 7.43-7.35 (m, 2H), 3.67 (s, 1H), 2.71 (s, 6H).

349-88-2
314 suppliers
$6.00/5g

124-40-3
545 suppliers
$18.00/100ml

383-31-3
56 suppliers
$12.00/100mg
Yield:383-31-3 100%
Reaction Conditions:
with triethylamine in tetrahydrofuran;water at 20; for 0.333333 h;
Steps:
90.1
(1) 4-Fluoro-1-(N,N-dimethylaminosulfonyl)benzene To a solution of 4-fluorobenzenesulfonyl chloride (1.95 g, 10.0 mmol) in THF (40 mL) were added triethylamine (2.09 mL, 15.0 mmol) and 50% aqueous dimethylamine solution (1.26 mL, 14.0 mmol) at room temperature, and the solution was stirred at the same temperature for 20 minutes. The reaction mixture was diluted with ethyl acetate, and washed with water. The organic layer was dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to obtain 4-fluoro-1-(N,N-dimethylaminosulfonyl)benzene (2.03 g, 10.0 mmol).yield: quantitative
References:
EP1354882,2003,A1 Location in patent:Page/Page column 85
110-18-9
570 suppliers
$10.00/5mL

349-88-2
314 suppliers
$6.00/5g

383-31-3
56 suppliers
$12.00/100mg