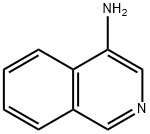
4-Aminoisoquinoline synthesis
- Product Name:4-Aminoisoquinoline
- CAS Number:23687-25-4
- Molecular formula:C9H8N2
- Molecular Weight:144.17

881668-81-1

23687-25-4
A mixture of N-benzyl-4-aminoisoquinoline (1.00 g, 4.27 mmol) was reacted with a mixture of acetic acid (30 mL) and H2SO4 (7.5 mL, concentration not specified) for 6 hours at 100 °C with stirring. Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched with saturated aqueous NaHCO3 solution. Subsequently, Na2CO3 (200 mL) was added and diluted with water, followed by extraction with EtOAc (5 x 25 mL). The organic phases were combined, concentrated and the residue was purified by chromatography (eluent: EtOAc) to afford the target compound 4-aminoisoquinoline (325 mg, 53% yield) as a yellow solid.1H NMR (DMSO-d6, 400 MHz) δ 8.49 (s, 1H), 8.10 (d, 1H), 7.90 (d, 1H), 7.87 (s, 1H ), 7.64-7.54 (m, 2H), 5.82 (s, 2H).
1532-97-4
359 suppliers
$14.00/10g

23687-25-4
226 suppliers
$24.00/1g
194032-33-2
84 suppliers
$195.00/1g
Yield:-
Steps:
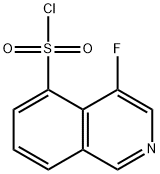
R.4 4-Fluoro-5-isoquinolinesulfonyl chloride
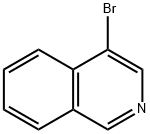
Referential Example 4 4-Fluoro-5-isoquinolinesulfonyl chloride From 4-bromoisoquinoline (75.0 g), 4-aminoisoquinoline (39.8 g) was synthetically obtained following J. Am. Chem. Soc., 64, 783 (1942).
References:
Hidaka, Hiroyoshi EP1074545, 2001, A1

1532-97-4
359 suppliers
$14.00/10g

23687-25-4
226 suppliers
$24.00/1g
36073-93-5
59 suppliers
inquiry

23687-25-4
226 suppliers
$24.00/1g
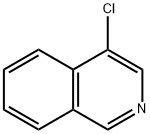
1532-91-8
88 suppliers
$41.00/1g

23687-25-4
226 suppliers
$24.00/1g