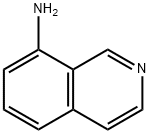
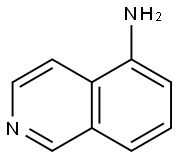
8-Aminoisoquinoline synthesis
- Product Name:8-Aminoisoquinoline
- CAS Number:23687-27-6
- Molecular formula:C9H8N2
- Molecular Weight:144.17
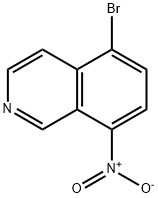
63927-23-1

23687-27-6
General procedure for the synthesis of 8-aminoisoquinolines from 5-bromo-8-nitroisoquinolines: 5-bromo-8-nitroisoquinolines (1.00 g, 3.95 mmol) were dissolved in methanol (70 mL), and hydrogenation was carried out by adding palladium/activated charcoal (10% Pd, 200 mg) as a catalyst for 18 hr at room temperature and atmospheric pressure. Upon completion of the reaction, the reaction mixture was filtered through Celite to remove the catalyst. The filtrate was concentrated and purified by column chromatography (eluent: ethyl acetate/heptane) to afford the target compound 8-aminoisoquinoline (100 mg, 18% yield) as a greenish-brown solid.1H NMR (DMSO-d6, 400 MHz) δ 9.43 (s, 1H), 8.32 (d, 1H), 7.54 (d, 1H), 7.40 (dd, 1H) , 6.99 (d, 1H), 6.72 (d, 1H), 6.22 (s, 2H).
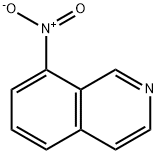
7473-12-3
17 suppliers
inquiry

23687-27-6
188 suppliers
$11.00/100mg
Yield:23687-27-6 99%
Reaction Conditions:
with C20H32Cl4Cu2N4O4 in water at 60; for 1 h;Inert atmosphere;Schlenk technique;
Steps:
2.4. General procedure for the reduction of nitro compounds withcopper complex catalyst
General procedure: Copper complex (0.001 mmol, 1mol%), appropriate nitro compounds (0.1 mmol, 1.0 equiv) and NaBH4 (10.0 mmol, 10 equiv)in water (2.0 mL) were charged into a 15 mL pressure tube under air atmosphere. Subsequently, the resulting mixture was stirred atroom temperature in closed vessel. After completion of the reaction(monitored by TLC), the crude reaction mixturewas extraction with ether (3 2 mL), and analyzed by GC-MS chromatography using n-dodecaneas an internal standard.
References:
Du, Jun;Gao, Li-Li;Jia, Wei-Guo;Li, Mei;Zhi, Xue-Ting [Journal of Molecular Structure,2020,vol. 1217]

63927-23-1
191 suppliers
$7.00/250mg

23687-27-6
188 suppliers
$11.00/100mg
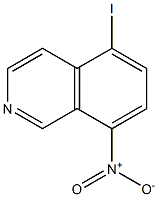
401916-13-0
10 suppliers
inquiry

23687-27-6
188 suppliers
$11.00/100mg
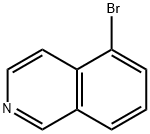
34784-04-8
354 suppliers
$7.00/1g

23687-27-6
188 suppliers
$11.00/100mg
1125-60-6
246 suppliers
$6.00/1g

23687-27-6
188 suppliers
$11.00/100mg