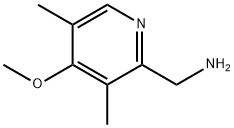
(4-methoxy-3,5-dimethylpyridin-2-yl)methanamine synthesis
- Product Name:(4-methoxy-3,5-dimethylpyridin-2-yl)methanamine
- CAS Number:130000-78-1
- Molecular formula:C9H14N2O
- Molecular Weight:166.22
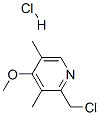
86604-75-3
562 suppliers
$10.00/1g

130000-78-1
19 suppliers
$286.00/500mg
Yield:130000-78-1 76%
Reaction Conditions:
with ammonia in methanol at 100; for 15 - 16 h;Heating / reflux;
Steps:
89.3.1; 219.1
Method 3 Step 1 : (4-Methoxy-3,5-dimethyl-pyridin-2-yl)-methylamine A solution OF 2-CHLOROMETHYL-4-METHOXY-3, 5-dimethyl-pyridine hydrochloride in 7N methanolic ammonia was placed in a pressure vessel and to 100 °C for 16 h. Concentration and flash chromatography gave the title compound as a greenish solid (76% yield). HPLC Rt: 3.773 MIN. 1H-NMR (CDC13) : 8 8. 19 (s, 1H), 4.35 (s, 2H), 3.76 (s, 3H), 2.24 (s, 3H), 2.18 (s, 3H).; STEPL : SYNTHESISOF2-AMINOMETHYL-4-METHOXY-3, 5-dimethylpyridine: A solution OF 2-CHLOROMETHYL-4-METHOXY-3, 5-dimethyl-pyridine HC1 (Aldrich 3.7g, 16.6 mmole) in 7N NH3/MeOH (Aldrich, 200MLS) was refluxed in a steel bomb for 15 hours. Removed the solvent under reduced pressure, the residue was taken into 5% MEOH/CH2CL2 and filtering it through a thin layer of silica gel afforded the product at 76% yield. HPLC RT was 2. 850 MIN. IHNMR (CDC13) 6 8. 18 (s, 1H), 4.32 (s, 2H), 3.76 (s, 3H), 2.23 (s, 3H), 2. 18 (s, 3H).
References:
WO2005/28434,2005,A2 Location in patent:Page/Page column 302; 366
![1H-Isoindole-1,3(2H)-dione, 2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-](/CAS/20210305/GIF/946071-49-4.gif)
946071-49-4
0 suppliers
inquiry

130000-78-1
19 suppliers
$286.00/500mg
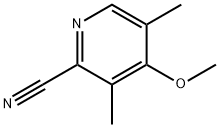
104916-41-8
13 suppliers
inquiry

130000-78-1
19 suppliers
$286.00/500mg