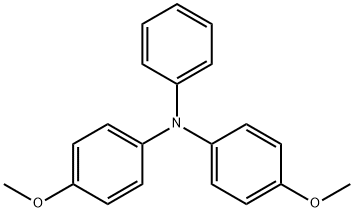
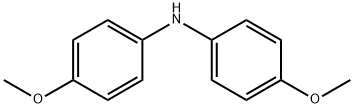
4-Methoxy-N-(4-methoxyphenyl)-N-phenylbenzenamine synthesis
- Product Name:4-Methoxy-N-(4-methoxyphenyl)-N-phenylbenzenamine
- CAS Number:20440-94-2
- Molecular formula:C20H19NO2
- Molecular Weight:305.37
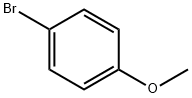
104-92-7

62-53-3

20440-94-2
To a 1L three-neck flask were added 18.63 g (0.2 mol) of aniline, 78.55 g (0.42 mol) of 4-bromoanisole, 56.10 g (0.5 mol) of potassium tert-butanol and 465.7 g of toluene. Subsequently, 3.66 g (4.0 x 10^-3 mol) of Pd2(dba)3 and 2.32 g (8.0 x 10^-3 mol) of (t-Bu)3PH-BF4 were added as catalysts. The reaction mixture was heated to 100~108°C for 4 hours. After completion of the reaction, the reaction solution was filtered and the filtrate was washed with 200 g of water and dried with anhydrous sodium sulfate. Purification by silica gel column chromatography using toluene as eluent gave a brown viscous liquid. The above crude product was recrystallized with toluene and ethanol (mass ratio 1:2) to afford 51.0 g of 4,4'-dimethoxytriphenylamine in yellow powder form in 83.5% yield.
5720-07-0
468 suppliers
$6.00/5g

62-53-3
688 suppliers
$10.00/1g

20440-94-2
87 suppliers
$34.00/100mg
Yield:20440-94-2 99%
Reaction Conditions:
with iron(III) trichloride hexahydrate;triphenylphosphine;sodium hydroxide in lithium hydroxide monohydrate;N,N-dimethyl-formamide at 20; for 0.75 h;Sonication;Green chemistry;
Steps:
Method C:
General procedure: The ultrasonic probe was immersed directlyin the reactor. An ultrasonic generator (Sonics VC 505 300W) emitted sound vibration into the reaction mixture. Sonificationwas achieved at a low frequency of 20 kHz (50% amplitude) at room temperature for 45 min. The aryl halideor arylboronic acids (4.5 mmol), the aniline compound (2.25mmol), the ligand (0.05 mmol) and the catalyst FeCl3.6H2O,Ag, CuO or ZrO2) (0.025 mmol) were placed in a reactor. 3mL of solvent were added. After the reaction, the mixturewas extracted (three times) with diethyl ether. The latter wasdried on MgSO4 and the solvent removed under vacuum.The coupling product was finally isolated with silica gelchromatography. The reaction yields were determined withgas chromatography on a Shimadzu 2014-GC chromatograph. The capillary column was DB-5 and the carrier gaswas helium.
References:
Said, Khemais;Salem, Ridha Ben [Letters in Organic Chemistry,2022,vol. 19,# 8,p. 627 - 635]

104-92-7
433 suppliers
$10.00/10g

62-53-3
688 suppliers
$10.00/1g

20440-94-2
87 suppliers
$34.00/100mg
696-62-8
358 suppliers
$5.00/1g

62-53-3
688 suppliers
$10.00/1g

20440-94-2
87 suppliers
$34.00/100mg

108-86-1
523 suppliers
$10.00/5g
101-70-2
220 suppliers
$32.00/1g

20440-94-2
87 suppliers
$34.00/100mg

5720-07-0
468 suppliers
$6.00/5g

62-53-3
688 suppliers
$10.00/1g
1208-86-2
166 suppliers
$8.00/1g

20440-94-2
87 suppliers
$34.00/100mg