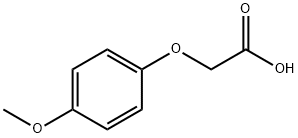
4-Methoxyphenoxyacetic acid synthesis
- Product Name:4-Methoxyphenoxyacetic acid
- CAS Number:1877-75-4
- Molecular formula:C9H10O4
- Molecular Weight:182.17
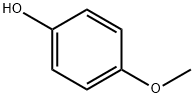
150-76-5
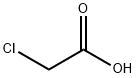
79-11-8

1877-75-4
Compounds B1-7 were prepared by similar methods. In the synthesis of B1, monochloroacetic acid (0.04 mol, 3.78 g) was first dissolved in 15 mL of deionized water under stirring and ice bath conditions. Subsequently, 25% NaOH solution was added drop by drop and the pH was adjusted to 9-10 to produce sodium chloroacetate solution. In another vessel, NaOH (0.03 mol, 1.20 g), 15 mL of deionized water, 5 mL of ethanol and phenol (0.04 mol, 3.76 g) were mixed, and the above sodium chloroacetate solution was added slowly with stirring. After addition, stirring was continued for 20 minutes, followed by dropwise addition of sodium chloroacetate solution and the reaction mixture was heated to 105 °C and refluxed for 5 hours. After completion of the reaction, the mixture was cooled to room temperature and acidified with dilute hydrochloric acid to pH 1-2. The precipitate was collected by filtration, washed several times with dilute hydrochloric acid, and after recrystallization and drying in vacuum, the white solid product phenoxyacetic acid (B1) was obtained.
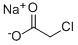
3926-62-3
300 suppliers
$16.69/250g

150-76-5
817 suppliers
$5.00/5 g

1877-75-4
147 suppliers
$7.00/250mg
Yield:1877-75-4 75%
Reaction Conditions:
Stage #1:4-methoxy-phenol with sodium hydroxide in ethanol;water at 20; for 0.333333 h;
Stage #2:sodium monochloroacetic acid in ethanol;water at 102; for 5 h;
Steps:
Synthesis of phenoxyacetic acid derivative (b1-7)
General procedure: 55mmol monochloroacetic acid was dissolved in 15mL deionized water under the condition of ice water bath, then 30% NaOH solution was used to adjust pH 8-9, sodium chloroacetate solution was obtained. 45mmol NaOH was dissolved in mixed solvent of 15mL deionized water and 5mL ethanol at room temperature with constant stirring, 45mmol phenol was subsequent slowly added. After stirring for another 20min, sodium chloroacetate solution was added. Subsequently, the mixture was refluxed at 102°C for 5h. After the mixture was cooled to room temperature, pH was adjusted to 1-2 with 2.0mol·L-1 HCl, amounts of white precipitations were gained. The precipitations were filtered and washed 3 times with dilute hydrochloric acid, dried at 60°C. White crude product was dispersed in 100mL heated deionized water, pH was adjusted to 8.0 using saturated potassium carbonate solution, then mixture solution was filtered, and filtrate was collected. White precipitated was obtained by adjusting pH of filtrate to 1-2 with 2.0mol·L-1 HCl. After cooled down to room temperature naturally, the mixture was filtered, washed with dilute hydrochloric acid, dried overnight in vacuum, then target product (b1) was obtained. The synthetic procedures of phenoxyacetic acid derivative (b2-7) were similar to that of phenoxyacetic acid (b1).
References:
Li, Dewei;Xiong, Suhao;Guo, Tiantong;Shu, Dehua;Xiao, Haihua;Li, Guizhi;Guo, Dongcai [Dyes and Pigments,2018,vol. 158,p. 28 - 35]
18598-23-7
14 suppliers
$395.00/25g

1877-75-4
147 suppliers
$7.00/250mg

150-76-5
817 suppliers
$5.00/5 g

1877-75-4
147 suppliers
$7.00/250mg

79704-02-2
16 suppliers
inquiry

1877-75-4
147 suppliers
$7.00/250mg