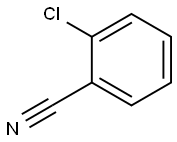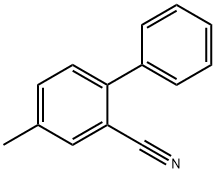
4-Methyl-[1,1'-biphenyl]-2-carbonitrile synthesis
- Product Name:4-Methyl-[1,1'-biphenyl]-2-carbonitrile
- CAS Number:64113-85-5
- Molecular formula:C14H11N
- Molecular Weight:193.24
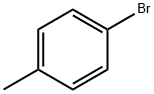
106-38-7
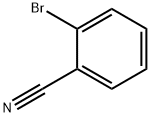
2042-37-7
![4-Methyl-[1,1'-biphenyl]-2-carbonitrile](/CAS/20180601/GIF/64113-85-5.gif)
64113-85-5
Step A: General procedure for the synthesis of 4-methyl-[1,1'-biphenyl]-2-carbonitrile from p-bromotoluene and 2-bromobenzonitrile: 1. To a solution of p-bromotoluene (30 g) in anhydrous ether (150 ml) was slowly added a pentane solution of t-BuLi (1.7 M, 210 ml) through a dropping funnel at -78 °C for 1 h 30 min. 2. the cooling bath was removed and the reaction mixture was continued to be stirred at room temperature for 2 hours. 3. The reaction solution was slowly transferred to a premixed ZnCl2 ether solution (1M, 180 ml) and anhydrous THF (360 ml) via cannula at room temperature. 4. The mixture was stirred at room temperature for 2 h. After stirring, the slurry was transferred via cannula to a solution of anhydrous THF (300 ml) containing 2-bromobenzonitrile (21.3 g) and NiCl2(Ph3P)2 (2.1 g). 5. The reaction mixture was stirred overnight (18 hrs) at room temperature and then slowly poured into ice-cold 1N HCl (1500 ml) while maintaining stirring. 6. The organic layer was separated and the aqueous phase was extracted with ether (3 x 300 ml). The organic layers were combined and washed sequentially with water and brine, then dried with MgSO4. 7. The solvent was removed under pressure to give the crude product (34 g) as a semi-solid. 8. The crude product was purified by rapid column chromatography on silica gel with ethyl acetate/hexane (1:12) as eluent to afford the target product 4-methyl-[1,1'-biphenyl]-2-carbonitrile (28 g, 88% yield) as a low melting point solid. NMR (CDCl3): δ 2.42 (s, 3H), 7.2-7.8 (m, 8H); FAB-MS: m/e 194 (M + H).
Yield:64113-85-5 94%
Reaction Conditions:
with potassium carbonate in 5,5-dimethyl-1,3-cyclohexadiene;
Steps:
2 Example 2
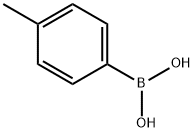
Example 2 165 mmol of bromobenzonitrile, 247 mmol of 4-methylphenylboronic acid, 330 mmol of potassium carbonate are heated with 0.2 mol % of trans-di-acetato-bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II) in 300 ml of xylene for 16 hours at 130° C. The reaction solution is distilled after aqueous workup. Yield: 94% of 2-cyano-4-methylbiphenyl.
References:
US5559277,1996,A