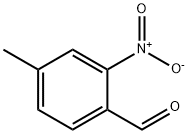
4-Methyl-2-nitrobenzaldehyde synthesis
- Product Name:4-Methyl-2-nitrobenzaldehyde
- CAS Number:20357-22-6
- Molecular formula:C8H7NO3
- Molecular Weight:165.15
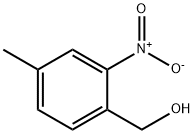
22996-24-3

20357-22-6
General procedure for the synthesis of 4-methyl-2-nitrobenzaldehyde from (4-methyl-2-nitrophenyl)methanol: (4-methyl-2-nitrophenyl)methanol (1.028 g, 6.15 mmol), pyridinium dichromate (3.47 g, 9.22 mmol), and molecular sieves (7 g, 6.15 mmol) were dissolved in anhydrous dichloromethane (14 mL) under argon protection and stirred at room temperature overnight. The reaction mixture was filtered through a silica/diatomaceous earth (1:1) column and subsequently extracted with water and dichloromethane. The organic layers were combined and the solvents were evaporated to give a light yellow solid product (0.665 g, 65.5% yield), which could be used in subsequent reactions without further purification. The product was characterized by 1H-NMR (CDCl3): δ 2.54 (s, 3H), 7.57-7.60 (m, 1H), 7.86-7.90 (m, 2H), 10.49 (s, 1H). the LC/MS analysis showed a retention time of 3.01 min (100% purity, no ionization peaks observed).

22996-24-3
59 suppliers
inquiry

20357-22-6
78 suppliers
$21.00/100mg
Yield: 100%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20; for 1 h;Cooling with ice;
Steps:
A.i.3 3 Synthesis of compound TB-31-4
Accurately weigh compound TB-31-3 (600mg, 3.59mmol) into the reaction flask, dissolve it with 15ml of dichloromethane, add Dess-martin (3.35g, 7.9mmol) to the reaction flask under ice bath, and after the addition, at room temperature For the reaction, the reaction liquid was reacted to completion of the raw materials after 1 hour. The reaction was quenched by adding sodium thiosulfate aqueous solution, the pH was adjusted to 7-8 with sodium bicarbonate, a small amount of DCM was added and the mixture was stirred until the layers were separated, DCM was extracted, the DCM layer was collected, dried and concentrated to obtain 620 mg of brown liquid, which became a solid after standing. The rate is 100%.
References:
Xihua University;Chengdu University Of Traditional Chinese Medicine;Qian Shan;Cao Zhixing;Xu Shijun;Li Yuzhi;Wang Zhouyu;Li Guobo;Yang Lingling CN111689901, 2020, A Location in patent:Paragraph 0135-0136; 0141-0142

50-00-0
896 suppliers
$10.00/25g
89-62-3
224 suppliers
$13.00/25g

20357-22-6
78 suppliers
$21.00/100mg
1455471-51-8
0 suppliers
inquiry
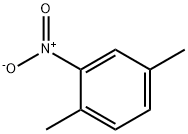
89-58-7
143 suppliers
$7.00/5g
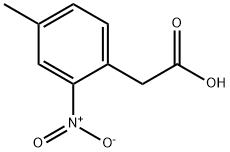
29640-90-2
11 suppliers
inquiry

20357-22-6
78 suppliers
$21.00/100mg

89-62-3
224 suppliers
$13.00/25g

20357-22-6
78 suppliers
$21.00/100mg
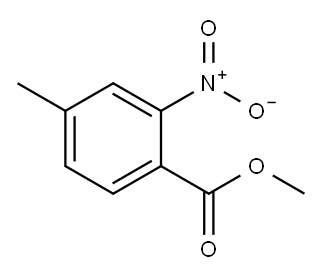
20587-29-5
4 suppliers
inquiry

20357-22-6
78 suppliers
$21.00/100mg