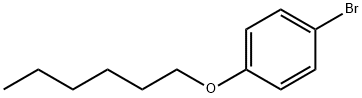
4-N-HEXYLOXYBROMOBENZENE synthesis
- Product Name:4-N-HEXYLOXYBROMOBENZENE
- CAS Number:30752-19-3
- Molecular formula:C12H17BrO
- Molecular Weight:257.17
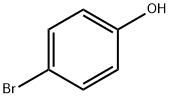
106-41-2

111-25-1

30752-19-3
Example 3.1 Synthesis of 1-bromo-4-(hexyloxy)benzene: Increase the temperature of the oil bath to 65 °C. In a 500 mL round-bottomed flask fitted with a stirrer, 10 g (57.8 mmol, 1.0 eq.) of 4-bromophenol and 26.36 g (190.7 mmol, 3.3 eq.) of potassium carbonate were added sequentially, followed by 202.3 mL of acetone. The reaction vessel was sealed, a condenser tube was fitted and purged continuously with argon for 10 min. After completion of the purge, 8.9 mL (64 mmol, 1.1 eq.) of bromohexane was slowly added via syringe, and the reaction mixture was stirred under reflux conditions in an oil bath for 24 hours. The progress of the reaction was monitored by thin layer chromatography (silica gel plate, hexane as the unfolding agent) and after confirming the completion of the reaction, the crude reaction mixture was filtered to remove potassium carbonate. Water was added to the filtrate and extracted twice with ether. The ether extracts were combined and dried with magnesium sulfate, followed by filtration to remove the desiccant. The solvent was removed by distillation under reduced pressure and the crude product obtained was purified by silica gel column chromatography (eluent hexane) to give 13.96 g (93.9% yield) of the target product 1-bromo-4-(hexyloxy)benzene as a clear liquid.GC-MS analysis showed the correct molecular ion peaks.1H NMR (500 MHz, CDCl3, internal standard TMS, δ 7.26 ppm): δ 7.355 (m, 2H), 6.770 (m, 2H), 3.912 (t, J = 6.75 Hz, 2H), 1.764 (m, 2H), 1.49-1.40 (m, 2H), 1.37-1.30 (m, 4H), 0.905 (t, J = 7.0 Hz, 3H).

106-41-2
498 suppliers
$13.00/5g

638-45-9
165 suppliers
$6.00/5g

30752-19-3
100 suppliers
$25.00/1 g
Yield:-
Reaction Conditions:
Stage #1:4-bromo-phenol with potassium carbonate in N,N-dimethyl-formamide at 70; for 0.5 h;
Stage #2:1-Iodohexane in N,N-dimethyl-formamide at 70;
Steps:
Synthesis of 4-Alkoxy-1-bromobenzene used for the synthesis of 7
General procedure: A mixture of 4-bromophenol (50 mmol),DMF(70ml) and potassium carbonate (100 mmol)was stirred at 70C for 0.5 h. To the suspension was added alkyl iodide (50 mmol) andthe mixture was stirred at 70C overnight. Water (200 ml) was added and the mixturewas extracted with hexane. The organic extracts were washed with brine (50 ml), 1NNaOH solution (50 ml) and water (100 ml), dried over MgSO4, and evaporated to give4-alkoxy-1-bromobenzene (> 90%).
References:
Uchida, Yoshiaki;Tamura, Rui;Suzuki, Katsuaki;Takahashi, Hiroki;Aoki, Yoshio;Nohira, Hiroyuki [Molecular Crystals and Liquid Crystals,2015,vol. 615,# 1,p. 89 - 106]

106-41-2
498 suppliers
$13.00/5g

111-25-1
321 suppliers
$10.00/5g

30752-19-3
100 suppliers
$25.00/1 g

111-25-1
321 suppliers
$10.00/5g
589-87-7
540 suppliers
$10.00/1g

30752-19-3
100 suppliers
$25.00/1 g
1132-66-7
15 suppliers
inquiry

30752-19-3
100 suppliers
$25.00/1 g
106-37-6
478 suppliers
$9.00/5g

111-27-3
547 suppliers
$5.00/25g

30752-19-3
100 suppliers
$25.00/1 g