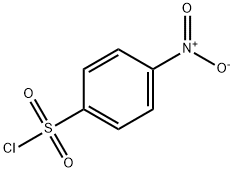
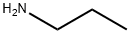4-Nitro-N-propylbenzenesulfonaMide, 97% synthesis
- Product Name:4-Nitro-N-propylbenzenesulfonaMide, 97%
- CAS Number:23530-47-4
- Molecular formula:C9H12N2O4S
- Molecular Weight:244.27
Yield:23530-47-4 96%
Reaction Conditions:
with pyridine in dichloromethane at 20;
Steps:
15 4.6.1. General procedure A (step 1)
General procedure: 4-Nitrobenzenesulfonyl chloride (12a, 1.8 mmol, 1 eq) or 4-nitrobenzoyl chloride (12b, 1.8 mmol, 1 eq) in dry DCM (25 mL) was added to a mixture of an appropriate amine (1.1-1.6 eq) and pyridine (1-1.6 eq) in dry DCM (25 mL). The reaction mixture was stirred at a sufficient temperature (rt to refluxing). When TLC indicated the disappearance of the starting material, the mixture was washed with 1 M HCl and the organic phase was dried with Na2SO4. The solvent was finally evaporated and the product was purified with column chromatography if needed. 4.6.4.15
4-Nitro-N-propylbenzenesulfonamide (13o)
From 12a (1.01 g, 4.6 mmol), n-propylamine (0.60 ml, 7.3 mmol) and pyridine (0.60 ml, 7.5 mmol) with general procedure A.
Stirred at rt for overnight.
Yellow solid (1.07 g, 96%) and it was used without further purification. 1H NMR (CDCl3): δ 0.89 (3H, t, J = 7.4 Hz), 1.52 (2H, m), 3.00 (2H, m), 4.78 (1H, t, J = 5.5 Hz), 8.07 (2H, d, J = 8.8 Hz), 8.37 (2H, d, J = 8.8 Hz).
References:
Yrj?l?, Sari;Parkkari, Teija;Navia-Paldanius, Dina;Laitinen, Tuomo;Kaczor, Agnieszka A.;Kokkola, Tarja;Adusei-Mensah, Frank;Savinainen, Juha R.;Laitinen, Jarmo T.;Poso, Antti;Alexander, Amy;Penman, June;Stott, Lisa;Anskat, Marie;Irving, Andrew J.;Nevalainen, Tapio J. [European Journal of Medicinal Chemistry,2016,vol. 107,p. 119 - 132]