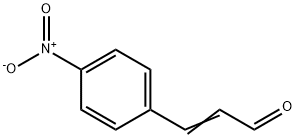
4-NITROCINNAMALDEHYDE synthesis
- Product Name:4-NITROCINNAMALDEHYDE
- CAS Number:1734-79-8
- Molecular formula:C9H7NO3
- Molecular Weight:177.16

64-17-5
826 suppliers
$10.00/50g
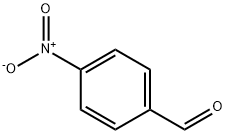
555-16-8
566 suppliers
$5.00/5g

1734-79-8
106 suppliers
$9.00/250mg
Yield:1734-79-8 90%
Reaction Conditions:
with caesium carbonate in water at 120; for 8 h;
Steps:
2.4. Procedure for synthesis of β-aryl enals and enones
General procedure: The β-aryl enals were synthesized by the following procedure.In a 50 mL round bottom (RB) flask, 2 mmol of benzaldehyde,9 mL ethanol (CH3CH2OH) and 3 mL water (H2O) was added in astoichiometric ratio of 3:1. After this, 2 equivalents of cesiumcarbonate (Cs2CO3) were added to the above mixture along with 25 mg of the synthesized CoCr2O4-HNT catalyst. The whole mixturewas then refluxed at 120 °C for 8 h. The reaction was monitoredthrough thin layer chromatography (TLC) and the productswere isolated through chromatography separation. Similarly, in a 50 mL RB flask b-aryl enones were synthesized bytaking the same amount of the substrate using 20 mg of CoCr2O4-HNT and in 1:3 ratio of H2O and ipr-OH (3 mL H2O + 9 mL ipr-OH).The reaction was stirred at room temperature for 4 h. After 4 h, thecatalyst was separated by normal filtration and the products wereseparated by chromatographic technique.
References:
Bania, Kusum K.;Baruah, Manash J.;Bhattacharyya, Pradip K.;Das, Biraj;Karunakar, Galla V.;Roy, Subhasish;Saikia, Lakshi;Saikia, Pinku;Sharma, Mukesh [Journal of Catalysis,2020,vol. 388,p. 104 - 121]

555-16-8
566 suppliers
$5.00/5g

75-07-0
428 suppliers
$14.00/5mL

1734-79-8
106 suppliers
$9.00/250mg

25798-60-1
2 suppliers
inquiry

1734-79-8
106 suppliers
$9.00/250mg
80793-24-4
9 suppliers
inquiry

1734-79-8
106 suppliers
$9.00/250mg
636-98-6
39 suppliers
$17.00/5g

107-18-6
0 suppliers
$24.60/100ml

1734-79-8
106 suppliers
$9.00/250mg

80793-24-4
9 suppliers
inquiry