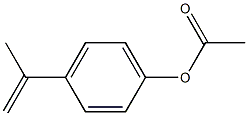
4-(prop-1-en-2-yl)phenyl acetate synthesis
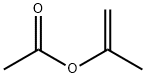
- Product Name:4-(prop-1-en-2-yl)phenyl acetate
- CAS Number:2759-56-0
- Molecular formula:C11H12O2
- Molecular Weight:176.2118

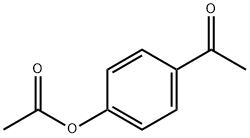
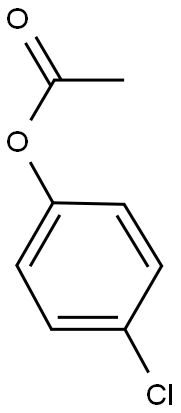
108-24-7
0 suppliers
$14.00/250ML
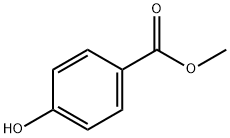
99-76-3
813 suppliers
$5.00/25g

74-88-4
357 suppliers
$15.00/10g

2759-56-0
3 suppliers
inquiry
Yield:2759-56-0 5 g
Reaction Conditions:
Stage #1: methyl 4-hydroxylbenzoate;methyl iodidewith magnesium in diethyl ether; for 2 h;Inert atmosphere;Reflux;
Stage #2: acetic anhydride for 10 h;Reflux;Inert atmosphere;
Steps:
4-(Prop-l-en-2-yl)phenyl acetate (SZV-1329/2)
4-(Prop-l-en-2-yl)phenyl acetate (SZV-1329/2) (Krimm H et al, DEI 193031) A solution of methyl 4-hydroxybenzoate (7.9 g, 52.0 mmol) in diethyl ether (90.0 ml) was added dropwise to a ~1M solution of methyl iodide (25.4 g, 179.0 mmol) and magnesium (4.2 g, 173.2 mmol) in diethyl ether (100.0 ml) under argon, upon intensive stirring and heating to reflux (within ~15 min) (Gilman H et al, J. Org. Chem., 1954, 19, 1067-1079). The reaction mixture was heated to reflux for 2 h, then was allowed to cool down to room temperature. A solution of NH4CI (20.3 g, 378.8 mmol) in H20 (63.0 ml) was carefully added dropwise upon intensive stirring. The two layers formed were separated, the aqueous layer was extracted with diethyl ether (2x40.0 ml). The combined organic layers were washed with saturated NaCl solution, dried (MgS04), filtered and evaporated to dryness to afford pale yellow solid (72%). The crude product (32.8 mmol) thus isolated was dissolved in acetic anhydride (11.8 ml) and the solution was heated to reflux for 10 h under argon. The reaction mixture was evaporated to dryness under reduced pressure (at max. 48-50 °C), the residue isolated was purified by flash chromatography on silica gel (w-hexane: EtOAc 9: 1). Pale yellow oil (5.0 g, 76%). NMR (400 MHz, Dimethyl sulfoxide-^) δ 7.53 (m, 2H, Ar-H), 7.10 (m, 2H, Ar-H), 5.41 (dq, J=1.4 and 0.8 Hz, 1H, =CH2), 5.10 (dq, J=1.4 and 1.4 Hz, 1H, =CH2), 2.27 (s, 3H, COCH3), 2.11 (dd, J=1.4, 0.8 Hz, 1H, =CH2).
References:
WO2015/87094,2015,A1 Location in patent:Page/Page column 142
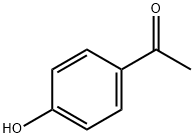
99-93-4
912 suppliers
$5.00/25g

2759-56-0
3 suppliers
inquiry