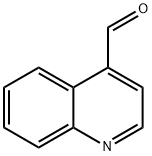
4-Quinolinecarboxaldehyde synthesis
- Product Name:4-Quinolinecarboxaldehyde
- CAS Number:4363-93-3
- Molecular formula:C10H7NO
- Molecular Weight:157.17
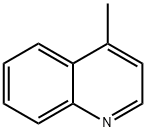
491-35-0

4363-93-3
The general procedure for the synthesis of 4-quinolinecarboxaldehyde from 4-methylquinoline was as follows: 5.0 g (35 mmol) of 4-methylquinoline and 5.0 g (45 mmol) of selenium dioxide were dissolved in toluene under argon protection and heated to reflux for 24 hours. After completion of the reaction, the reaction mixture was diluted with dichloromethane, washed sequentially with saturated saline and dried with anhydrous magnesium sulfate. After concentration under reduced pressure, the reaction was purified by silica gel column chromatography using a solvent mixture of hexane and ethyl acetate (4:1, v/v) as eluent to give 4.0 g (73% yield) of the target product 4-quinolinecarboxaldehyde as a solid. Nuclear magnetic resonance hydrogen spectroscopy (1H NMR) data: δ 10.54 (s, 1H), 9.22 (d, J = 4.3 Hz, 1H), 9.04 (d, J = 8.6 Hz, 1H), 8.24 (d, J = 8.2 Hz, 1H), 7.84 (t, J = 7.6 Hz, 1H), 7.81 (d, J = 4.3 Hz, 1H), 7.76 (t , J = 8.0 Hz, 1H). Nuclear magnetic resonance carbon (13C NMR) data: δ 193.1, 150.7, 149.5, 137.0, 130.4, 130.3, 129.6, 126.0, 124.7, 124.1. mass spectrometry (electrospray ionization) data: m/z 158.0 (100%) ([M + H]+), 130.2.
91-22-5
415 suppliers
$10.00/25g

4363-93-3
233 suppliers
$7.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: iron(II) perchlorate hydrate; dihydrogen peroxide / 0.17 h / 20 °C
2: Dess-Martin periodane / dichloromethane / 1 h / 0 - 20 °C
References:
Shantharjun, Bangarigalla;Vani, Damera;Unnava, Ramanjaneyulu;Sandeep, Mummadi;Reddy, Kallu Rajender [Organic and Biomolecular Chemistry,2021,vol. 19,# 3,p. 645 - 652]

491-35-0
252 suppliers
$10.00/10g

4363-93-3
233 suppliers
$7.00/250mg
6281-32-9
70 suppliers
$178.00/1g

4363-93-3
233 suppliers
$7.00/250mg
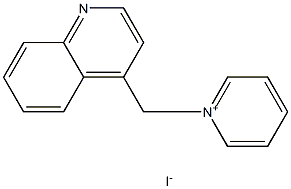
5450-80-6
1 suppliers
inquiry

4363-93-3
233 suppliers
$7.00/250mg
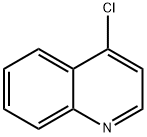
611-35-8
215 suppliers
$5.00/250mg
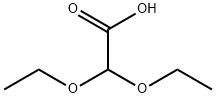
20461-86-3
33 suppliers
inquiry

4363-93-3
233 suppliers
$7.00/250mg