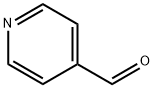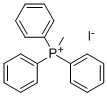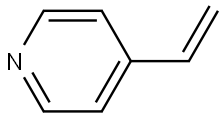
4-Vinylpyridine synthesis
- Product Name:4-Vinylpyridine
- CAS Number:100-43-6
- Molecular formula:C7H7N
- Molecular Weight:105.14
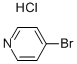
19524-06-2
483 suppliers
$6.00/5g
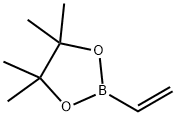
75927-49-0
212 suppliers
$6.00/1g

100-43-6
160 suppliers
$22.19/5ml
Yield:100-43-6 55.55%
Reaction Conditions:
with trans-bis(triphenylphosphine)palladium dichloride;potassium carbonate in 1,4-dioxane;water at 100;Inert atmosphere;
Steps:
S-19.1 Step 1 : Synthesis of 4-vinylpyridine.
To a stirred solution of 4-Bromopyridine hydrochloride (0.500 g, 2.577 mmol, 1.0 equiv) in Dioxane (8 mL) was added 2- Vinyl-4, 4, 5, 5- tetramethyl-l,3,2-dioxaoborolane (0.595 g, 3.865 mmol . l .Sequiv) and a solution of K2CO3 (0.71 1 g , 5.154 mmol, 2.0 equiv) in water (4 mL), and the mixture was purged with N2 gas for 10 min, followed by the addition of Pd(PPh3)Ch (0.090 g, 0.128 mmol. 0.05 equiv). The resulting reaction mixture was heated at 100° C for overnight. Product formation was confirmed by TLC. Reaction mixture was cooled to RT, diluted with water (50 mL) extracted with ethyl acetate ( 100 mL c 2). Combined organic extracts wore washed with water (50 mL c 2) & brine (50 mL), dried over anhydrous NarSOr and concentrated lire erode product ws purified by flash chromatography (0-15percent EA in hexane as an eluent) to obtain 4-Vinylpyridine (0.150 g, 55.55percent Yield) as a Semi-solid. NMR (400 MHz, CHLQRGFORM-ri) 5 8.42 - 8 68 (m, 2 H) 7.14 - 7.38 (m, 2 H) 6.66 (dd, 7=17 54, 10.96 Hz, 1 H) 5.97 (d, .7=17 54 Hz, 1 H) 5.48 (d, 7=10.52 Hz, 1 H).
References:
PRAXIS BIOTECH LLC;PUJALA, Brahmam;PANPATIL, Dayanand;BERNALES, Sebastian;BELMAR, Sebastian;URETA DÍAZ, Gonzalo Andrés WO2020/132661, 2020, A2 Location in patent:Paragraph 0192
108-89-4
344 suppliers
$15.00/25mL

50-00-0
896 suppliers
$10.00/25g

100-43-6
160 suppliers
$22.19/5ml

108-89-4
344 suppliers
$15.00/25mL

100-43-6
160 suppliers
$22.19/5ml
2629-72-3
146 suppliers
$10.00/1g

100-43-6
160 suppliers
$22.19/5ml