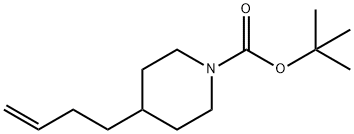
tert-butyl 4-(but-3-en-1-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(but-3-en-1-yl)piperidine-1-carboxylate
- CAS Number:403857-14-7
- Molecular formula:C14H25NO2
- Molecular Weight:239.35
Yield:403857-14-7 60%
Reaction Conditions:
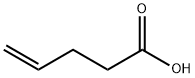
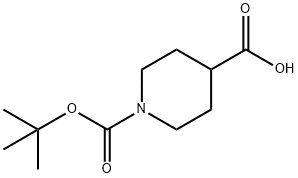
Stage #1: pent-4-enoic acid;N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acidwith 4-dimethylaminopyridine;NHPI;diisopropyl-carbodiimide in dichloromethane at 20; for 1 h;
Stage #2: with nickel(II) chloride ethylene glycol dimethyl ether complex;sodium iodide;2,6-di(4,5-dihydro-1,3-oxazol-2-yl)-4-methoxypyridine in dichloromethane;N,N-dimethyl-formamide at 20; for 2.5 h;Electrochemical reaction;
Steps:
General procedure
General procedure: The general procedure proceeds as follows: to a mixture of acid components (the less expensive of which is used in 3 equiv.) in CH2Cl2 are added diisopropylcarbodiimide (DIC) and N-hydroxyphthalimide (NHPI) (1.1 equiv. each relative to total acid quantity, 4.4 equiv. total) along with catalytic amount of 4-dimethylaminopyridine (10 mol% to total acid quantity, 0.4 equiv. total). After stirring for 1 h, without any solvent removal, the solution is diluted with N,N-dimethylformamide and NiCl2?dme along with L4 are added (roughly 5 mol% each relative to total acid quantity, 20 mol% total), followed by the addition of NaI (0.2 M). Electrolysis using a standard ElectraSyn2.0 potentiostat (Zn anode and Ni foam cathode) for about 2.5 h (0.1 mmol scale) followed by standard workup and purification delivers the coupled product.
References:
Zhang, Benxiang;Gao, Yang;Hioki, Yuta;Oderinde, Martins S.;Qiao, Jennifer X.;Rodriguez, Kevin X.;Zhang, Hai-Jun;Kawamata, Yu;Baran, Phil S. [Nature,2022,vol. 606,# 7913,p. 313 - 318]
2629-72-3
146 suppliers
$10.00/1g

403857-14-7
8 suppliers
inquiry
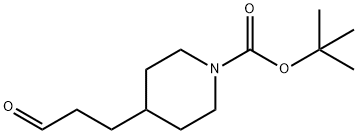
165528-85-8
26 suppliers
inquiry

403857-14-7
8 suppliers
inquiry
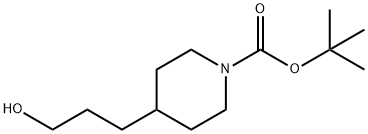
156185-63-6
131 suppliers
$24.00/250mg

403857-14-7
8 suppliers
inquiry
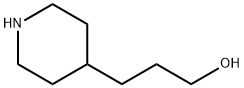
7037-49-2
135 suppliers
$19.00/250mg

403857-14-7
8 suppliers
inquiry