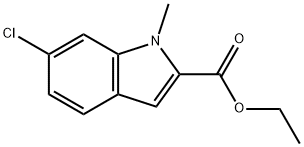
1-Methyl-6-chloro-1H-indole-2-carboxylic acid ethyl ester synthesis
- Product Name:1-Methyl-6-chloro-1H-indole-2-carboxylic acid ethyl ester
- CAS Number:43142-81-0
- Molecular formula:C12H12ClNO2
- Molecular Weight:237.68
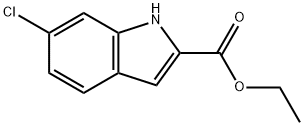
27034-51-1
145 suppliers
$6.00/250mg

74-88-4
356 suppliers
$15.00/10g

43142-81-0
6 suppliers
inquiry
Yield:43142-81-0 100%
Reaction Conditions:
Stage #1: ethyl 6-chloro-1H-indole-2-carboxylatewith sodium hydride in N,N-dimethyl-formamide at 20; for 1 h;Inert atmosphere;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20; for 4 h;Inert atmosphere;
Steps:
Ethyl 6-chloro-1-methyl-1H-indole-2-carboxylate (4)
To a suspension of sodium hydride (0.73 g, 29 mmol) in dry N,N-dimethylformamide(58 mL) was added 3 (6.1 g, 27.4 mmol) at room temperature and the reaction mixture was stirred under argon for 1 h before addition of methyl iodide (1.82 mL, 29 mmol).The reaction mixture was stirred at room temperature for 4 h and evaporated to give a residue which was redistributed in dichloromethane(200 mL) and water (300 mL). The organic layer was separated and washed twice with water (2 200 mL) before beingdried (Na2SO4). Evaporation followed by crystallization in ethanol gave 4 as beige crystals (6.5 g, quantitative). Rf (heptane/ethyl acetate,7/3): 0.52. Mp: 75-76 C. 1H NMR (CDCl3) d 7.56 (d, J 8.4 Hz,1H), 7.35 (d, J 1.6 Hz, 1H), 7.24 (s, 1H), 7.09 (d, J 8.4 Hz, 1H), 4.36(q4, J 6.8 Hz, 2H), 4.01 (s, 3H), 1.41 (t, J 6.8 Hz, 3H). 13C NMR(CDCl3) d 161.8 [C], 139.8 [C], 130.9 [C], 128.7 [C], 124.2 [C], 123.4[CH], 121.4 [CH], 110.1 [CH], 110.0 [CH], 60.6 [CH2], 31.7 [CH3], 14.3[CH3].
References:
Damont, Annelaure;Marguet, Frank;Puech, Frédéric;Dollé, Frédéric [European Journal of Medicinal Chemistry,2015,vol. 101,art. no. 8017,p. 736 - 745]
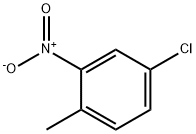
89-59-8
212 suppliers
$29.80/100g

43142-81-0
6 suppliers
inquiry
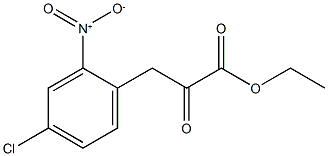
540523-98-6
3 suppliers
inquiry

43142-81-0
6 suppliers
inquiry
16732-75-5
150 suppliers
$14.00/250mg

43142-81-0
6 suppliers
inquiry
